How Are Density, Mass & Volume Related?
Relationship Between Mass, Density and Volume
Relationship Between Mass, Density and Volume
Density describes the ratio of mass to volume of an object or substance. Mass measures the resistance of a material to accelerate when a force acts upon it. According to Newton's second law of motion (F = ma), the net force acting upon an object equals the product of its mass times acceleration.
This formal definition of mass lets you put it in other contexts such as calculating energy, momentum, centripetal force and gravitational force. Since gravity is very nearly the same over the surface of the Earth, weight becomes a good indicator of mass. Increasing and decreasing the amount of material measured increases and decreases the mass of the substance.
TL;DR (Too Long; Didn't Read)
An object's density is the ratio of mass to volume of an object. The mass is how much it resists acceleration when a force is applied to it and generally means how much of an object or substance there is. Volume describes how much space an object takes up. These quantities can be used in determining pressure, temperature and other features of gases, solids and liquids.
There is a clear relationship between mass, density and volume. Unlike mass and volume, increasing the amount of material measured does not increase or decrease density. In other words, increasing the amount of freshwater from 10 grams to 100 grams will also change the volume from 10 milliliters to 100 milliliters but the density remains 1 gram per milliliter (100 g ÷ 100 mL = 1 g/mL).
This makes density a useful property in identifying many substances. However, since volume deviates with changes in temperature and pressure, density can also change with temperature and pressure.
Measuring Volume
Measuring Volume
For a given mass and **volume,** how much physical space a material takes up, of an object or substance, the density remains constant at a given temperature and pressure. The equation for this relationship is
\(\rho = \frac{m}{V}\)
in which ρ (rho) is density, m is mass and V is volume, making the density unit kg/m3. The reciprocal of density (1/ρ) is known as the specific volume, measured in m3 /kg.
Volume describes how much space a substance occupies and is given in liters (SI) or gallons (English). The volume of a substance is determined by how much material is present and how closely the particles of the material are packed together.
As a result, temperature and pressure can greatly affect the volume of a substance, especially gases. As with mass, increasing and decreasing the amount of material also increases and decreases the volume of the substance.
Relationship Between Pressure, Volume and Temperature
Relationship Between Pressure, Volume and Temperature
For gases, the volume is always equal to the container that the gas is inside. This means that, for gases, you can relate the volume to temperature, pressure and density using the ideal gas law
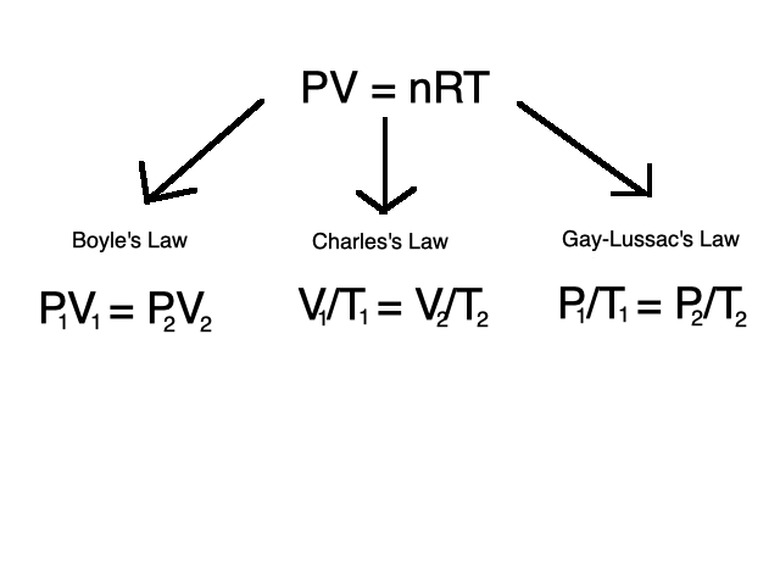
\(PV=nRT\)
in which P is pressure in atm (atmospheric units), V is volume in m3 (meters cubed), n is the number of moles of the gas, R is the universal gas constant (R = 8.314 J/(mol x K)) and T is temperature of the gas in Kelvin.
Three more laws describe the relationships among volume, pressure and temperature as they change when all other quantities are held constant. The equations are known as Boyle's Law, Gay-Lussac's Law and Charles's Law, respectively.
In each law, the left-hand variables describe volume, pressure and temperature at an initial point in time while the right-hand variables describe them at another later time point. Temperature is constant for Boyle's Law, volume is constant for Gay-Lussac's Law and pressure is constant for Charles's Law.
These three laws follow the same principles of the ideal gas law, but describe the changes in contexts of either temperature, pressure, or volume held constant.
The Meaning of Mass
The Meaning of Mass
Though people generally use mass to refer to how much of a substance is present or how heavy a substance is, the various ways people refer to masses of different scientific phenomena means that mass needs a more unified definition that encompasses all of its uses.
Scientists typically talk about subatomic particles, such as electrons, bosons or photons, as having a very small amount of mass. But the masses of these particles are actually just energy. While the mass of protons and neutrons are stored in gluons (the material that keeps protons and neutrons together), the mass of an electron is much more negligible given that electrons are about 2,000 times lighter than protons and neutrons.
Gluons account for the strong nuclear force, one of the four fundamental forces of the universe alongside electromagnetic force, gravitational force and the weak nuclear force, in keeping neutrons and protons bound together.
Mass and Density of the Universe
Mass and Density of the Universe
Though the size of the entire universe isn't exactly known, the observable universe, the matter in the universe that scientists have studied, has a mass of about 2 x 1055 g, about 25 billion galaxies the size of the Milky Way. This spans 14 billion light years including dark matter, matter that scientists aren't completely sure of what it's made of and luminous matter, what accounts for stars and galaxies. The universe's density is about 3 x 10-30 g/cm3.
Scientists come up with these estimates by observing changes in the Cosmic Microwave Background (artifacts of electromagnetic radiation from primitive stages of the universe), superclusters (clusters of galaxies) and Big Bang nucleosynthesis (production of non-hydrogen nuclei during the early stages of the universe).
Dark Matter and Dark Energy
Dark Matter and Dark Energy
Scientists study these features of the universe to determine its fate, whether it will continue to expand or at some point collapse in of itself. As the universe continues to expand, scientists used to think that gravitational forces give objects an attractive force between one another to slow the expansion.
But in 1998, the Hubble Space Telescope observations of distant supernovae showed that the universe was the universe's expansion has increased over time. Though scientists hadn't figured out what exactly was causing the acceleration, this expansion acceleration lead scientists to theorize that dark energy, the name for this unknown phenomena, would account for this.
There remain many mysteries about mass in the universe, and they account for most of the universe's mass. About 70% of the mass energy in the universe comes from dark energy and about 25% from dark matter. Only about 5% comes from ordinary matter. These detailed pictures of various types of masses in the universe show how varied mass can be in different scientific contexts.
Buoyant Force and Specific Gravity
Buoyant Force and Specific Gravity
The gravitational force of an object in water and the buoyant force that keeps it upward determine whether an object floats or sinks. If the object's buoyant force or density is greater than that of the liquid, it floats, and, if not, it sinks.
The density of steel is much higher than the density of water but shaped appropriately, the density may be reduced with air spaces, creating steel ships. The density of water being greater than the density of ice also explains why ice floats in water.
Specific gravity is the density of a substance divided by the reference substance's density. This reference is either air without water for gases or fresh water for liquids and solids.
Cite This Article
MLA
Ather, S. Hussain. "How Are Density, Mass & Volume Related?" sciencing.com, https://www.sciencing.com/density-mass-volume-related-6399069/. 27 December 2020.
APA
Ather, S. Hussain. (2020, December 27). How Are Density, Mass & Volume Related?. sciencing.com. Retrieved from https://www.sciencing.com/density-mass-volume-related-6399069/
Chicago
Ather, S. Hussain. How Are Density, Mass & Volume Related? last modified August 30, 2022. https://www.sciencing.com/density-mass-volume-related-6399069/