What Is "Direct Titration"?
Scientists rely on direct titration to find the quantity of a substance within a solution with chemical reactions. When performed correctly, this process can very accurately depict chemical quantities using specialized acids and laboratory glassware. For the titration to work properly, the last complex must form rapidly enough for the scientists to analyze it.
Definition
Definition
Direct titration is a way to determine the contents of a substance quantitatively. Scientists may be aware of a reactant, but not know the reactant's quantity. Direct titration is sometimes based on indicators that respond to the analyzed material, called the analyte. Other times, the methods are based on the use of added metal ions, which are individual atoms or molecules of a specific type of metal.
Ethylenediaminetetracetic Acid and the Potentiometric Method
Ethylenediaminetetracetic Acid and the Potentiometric Method
Technicians can perform titration using ethylenediaminetetracetic acid with metal-ion indicators. This method doesn't work in all situations, since the reaction is sometimes so slow that titration becomes unrealistic. The used metal ion must have less stability than the analyte. Another method of direct titration is the potentiometric method, used for endpoint detection with metal ions that have specific available electrodes. The endpoint is the point where the titration process ends.
Complexometric Titration
Complexometric Titration
For complexometric titration, scientists use aminopolycarboxylic acids to identify metals. Colored complexes form and the scientists use the data collected from this formation to determine the quantity of the analyte. The direct method of complexometric titration involves the use of a metal-salt solution titrated with a complexing compound solution. Complexing compound solutions contain atoms or compounds that form complexes with other atoms or compounds. Scientists find the equivalence point from an added indicator. The equivalence point is when the added titrant is stoichiometrically equal to the analyte. Stoichiometry involves chemical reactions balancing.
Burette Solution
Burette Solution
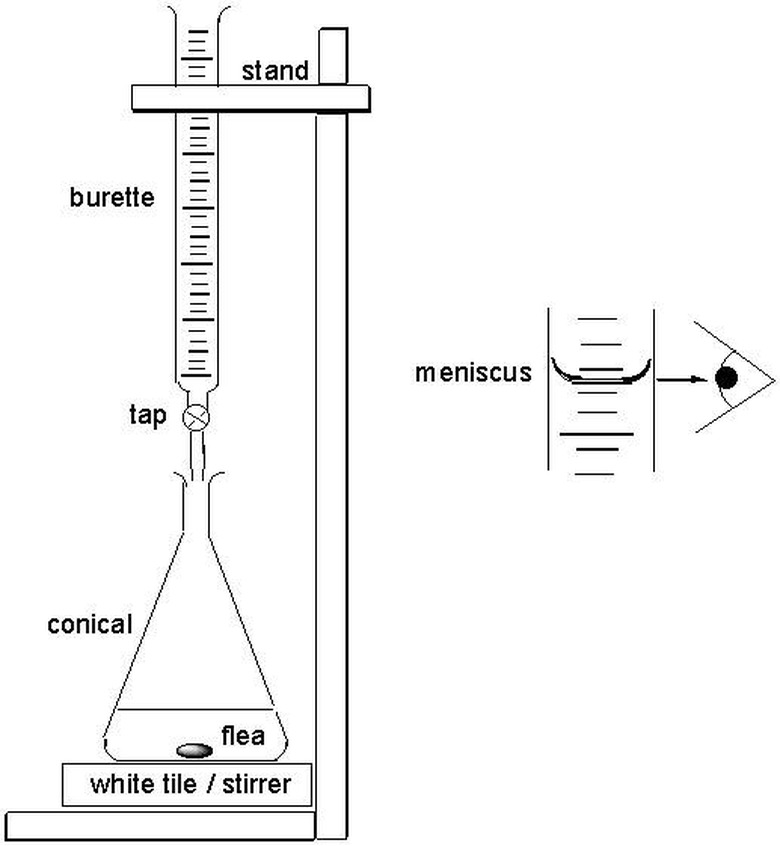
Direct titration is called "direct" because the scientist approaches the endpoint directly. The titrant enters the solution through drops from the burette so the final drop does not surpass the endpoint. With direct titration, scientists treat a soluble substance contained in a solution, which is contained in a vessel called the titrate. The standardized solution is called the titrant. The endpoint is determined instrumentally or visually with the help of an indicator. Scientists add the titrant to the correct burette, a vertical and cylindrical piece of glassware with a precision tap that releases small amounts of liquid at specific amounts. The scientists fill the burette to 30 to 100 percent capacity.
References
Cite This Article
MLA
Robert, Chuck. "What Is "Direct Titration"?" sciencing.com, https://www.sciencing.com/direct-titration-10018481/. 24 April 2017.
APA
Robert, Chuck. (2017, April 24). What Is "Direct Titration"?. sciencing.com. Retrieved from https://www.sciencing.com/direct-titration-10018481/
Chicago
Robert, Chuck. What Is "Direct Titration"? last modified August 30, 2022. https://www.sciencing.com/direct-titration-10018481/