Hydrous Vs. Anhydrous
In science, you may do experiments with hydrous and anhydrous compounds. The main difference between hydrous compounds and anhydrous compounds is the presence of water molecules. A hydrous compound contains water molecules, but an anhydrous compound contains none.
Hydrous Compound Properties
Hydrous Compound Properties
A hydrous compound (a hydrate) is a chemical compound with water in its structure. For example, hydrated salts have water within their crystals. Hydrates form naturally when ionic compounds are exposed to air and make bonds with water molecules. Specifically, the bond is formed between the cation of the molecule and the water molecule. The water that remains is usually known as water of hydration or water of crystallization. Most hydrates are stable at room temperature. However, some involuntarily surrender water in the atmosphere. These hydrates are known as efflorescent.
Anhydrous Compound Properties
Anhydrous Compound Properties
An anhydrous compound (an anhydrate) is a compound with no water in its structure. After water is removed from a hydrate, it becomes an anhydrate. The water molecules are removed by suction or heating the compound to a high temperature. For example, an anhydrous salt has had water driven out from its crystals. An anhydrous compound from a hydrate is generally highly soluble in water, and when dissolved in water it will be a similar color to that of the original hydrate, even if it changed color transforming from the hydrate to the anhydrous compound.
Examples of Hydrates
Examples of Hydrates
Examples of hydrates are gypsum (commonly used in the manufacturing of wallboard, cement and plaster of Paris), Borax (used in many cosmetic, cleaning and laundry products) and epsom salt (used as a natural remedy and exfoliant). Hydrates are often used in skin care products to infuse moisture into the body. The world contains many gas hydrates, crystalline solids in which gas molecules are enclosed in structures made of water molecules. These form from very low temperatures and high pressure. They are mixed with sediment and found primarily deep in the ocean and in Arctic regions.
Examples of Anhydrates
Examples of Anhydrates
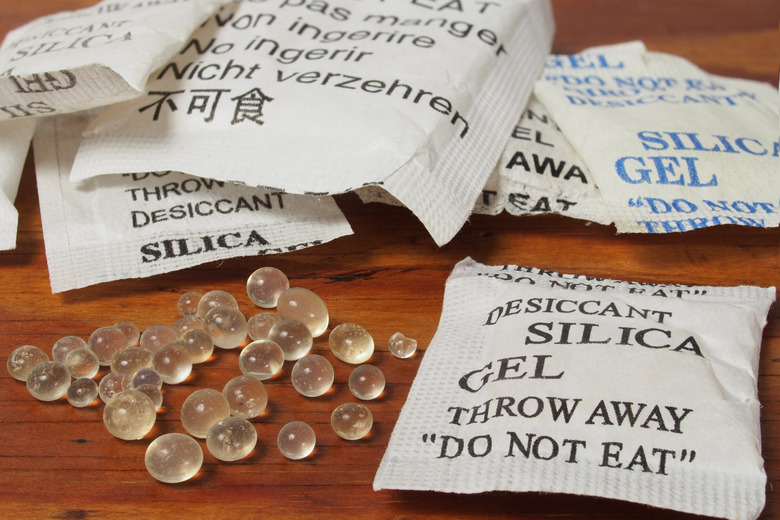
Anhydrates, also known as desiccants, remove water, so are often used in drying agents, such as paper products. Silica gel is one of the most commonly used anhydrates. A packet of silica gel is kept inside finished purses and other products to absorb water, keeping the surrounding area dry and preventing the growth of molds. Anhydrates can also retain moisture in food and tobacco products when they are in humectant form.
Cite This Article
MLA
Gillespie, Claire. "Hydrous Vs. Anhydrous" sciencing.com, https://www.sciencing.com/hydrous-vs-anhydrous-5554365/. 25 April 2018.
APA
Gillespie, Claire. (2018, April 25). Hydrous Vs. Anhydrous. sciencing.com. Retrieved from https://www.sciencing.com/hydrous-vs-anhydrous-5554365/
Chicago
Gillespie, Claire. Hydrous Vs. Anhydrous last modified March 24, 2022. https://www.sciencing.com/hydrous-vs-anhydrous-5554365/