How To Make Sodium Carbonate Solution
Sodium carbonate is an inorganic salt with the chemical formula Na2CO3. This compound, used in such industrial applications as glass production, as an electrolyte or as a component of toothpastes, also works as a cleaning agent. Prepare sodium carbonate solutions with a certain concentration, commonly expressed either as a mass percentage of the dissolved compound (for example, a 5 percent solution) or in molarity—the number of moles of such a substance per 1 L of the solution.
Making Sodium Carbonate
You can make sodium carbonate for these solutions yourself at home simply by heating sodium bicarbonate, or household baking soda. When you heat it to above 80 degrees Celsius (176 degrees Fahrenheit), the sodium bicarbonate breaks down into sodium carbonate, carbon dioxide and water vapor. For every 2 moles of sodium bicarbonate, you get 1 mole of sodium carbonate plus the CO2 gas and water; the bicarbonate powder seems to "shrink" as you bake it. You can heat the sodium bicarbonate in clean glassware or an aluminum pan.
Making Solutions With a Given Mass Percentage
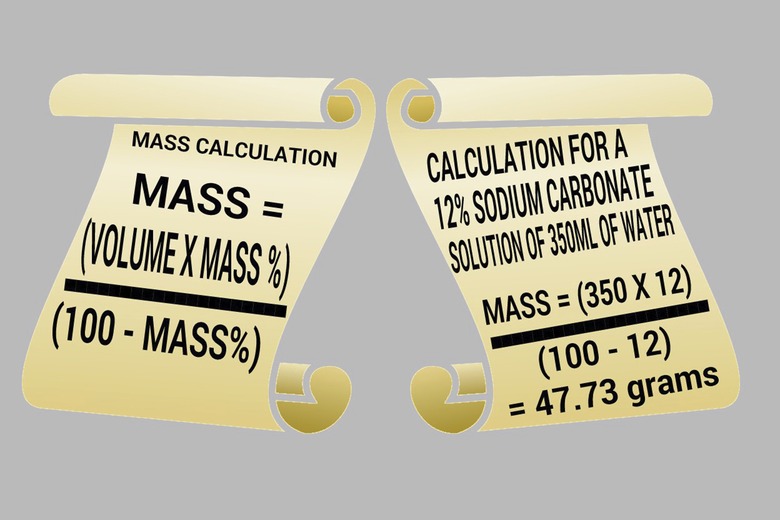
1. Calculate Reactants
Calculate the mass of sodium carbonate needed using the following formula: Mass = (volume x mass percentage) / (100 – mass percentage). For example, to make a 12 percent solution using 350 mL of water, use this equation to determine the amount of sodium carbonate to use: Mass = 350 x 12 / (100 – 12) = 47.73 g
2. Measure Sodium Carbonate
Weigh the calculated amount of sodium carbonate on the scale.
3. Prepare Solution
Pour water (350 Ll in our example) into the beaker, and add sodium carbonate.
4. Mix Solution
Mix the solution with the spoon or gently swirl the beaker until the salt dissolves completely.
Making Solutions with a Given Molarity
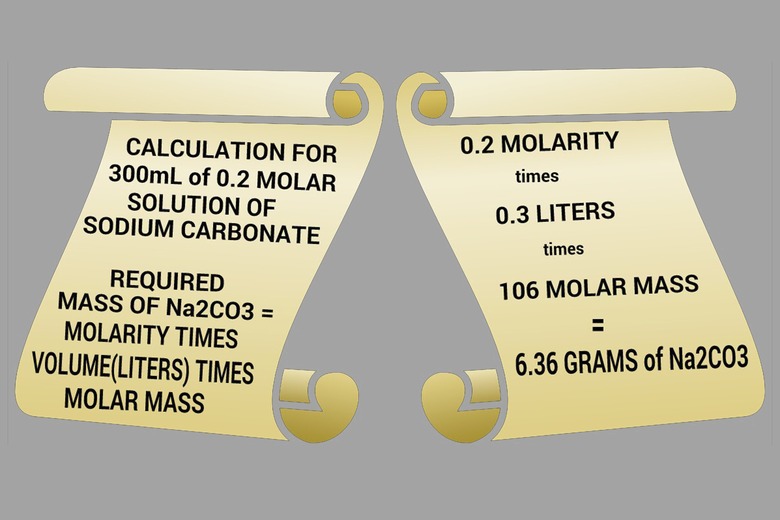
1. Determine Sodium Carbonate Needed
Multiply molarity by the solution volume (in liters) and the number 106—the molar mass of sodium carbonate—to calculate the mass of sodium carbonate needed. For example, to make 300 mL of 0.2 molar solution, you will need: 0.2 x 0.3 L x 106 = 6.36 g Note that 300 mL = 0.3 L
2. Weigh Sodium Carbonate
Weigh the calculated amount of sodium carbonate on the scale.
3. Add to Water
Pour distilled water—20 to 30 mL less than the final volume—into the beaker, then add sodium carbonate. In our example, start with 270 to 280 mL of water.
4. Stir Solution
Mix the solution with a spoon or gently swirl the beaker until the salt dissolves completely.
5. Measure Solution
Pour the solution into the graduated cylinder and fill to the final volume with distilled water.
Things Needed
- Sodium carbonate
- Scale
- Beaker
- Spoon
- Distilled water
- Graduated cylinder
References
- "Chemistry"; K.W. Whitten, R.E. Davis, L. Peck and G.G. Stanley; 2009
- "Sodium Carbonate: A Versatile Material"; T. Lister, C. Osborne and I. Bertin; 2000
- Rutgers University: Converting Sodium Bicarbonate to Sodium Carbonate
- Scientific American: Vanishing Baking Soda
Cite This Article
MLA
Writer, Contributing. "How To Make Sodium Carbonate Solution" sciencing.com, https://www.sciencing.com/make-sodium-carbonate-solution-5595471/. 27 April 2018.
APA
Writer, Contributing. (2018, April 27). How To Make Sodium Carbonate Solution. sciencing.com. Retrieved from https://www.sciencing.com/make-sodium-carbonate-solution-5595471/
Chicago
Writer, Contributing. How To Make Sodium Carbonate Solution last modified March 24, 2022. https://www.sciencing.com/make-sodium-carbonate-solution-5595471/