How To Find The Neutrons In The Periodic Table
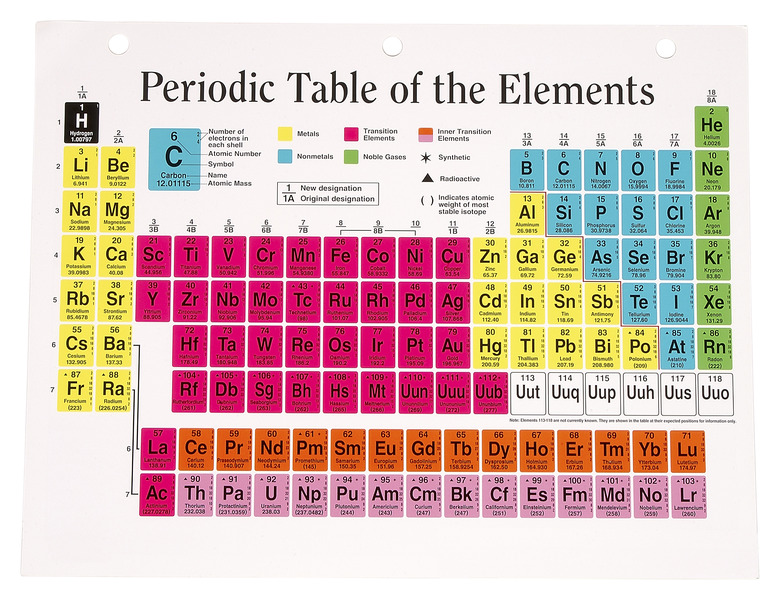
The periodic table lists every element on Earth and information about those elements. With this table, you can see how the elements relate to each other and how to find out how many particles are in an atom of each of them. An atom is made up of protons, electrons and neutrons.
Step 1
Choose an element and find it on the periodic chart. For this example, use gold, which is located in row six of the table (atomic sign: Au).
Step 2
Locate the atomic number and the atomic weight of the element. The atomic number is usually located in the top left hand corner of the box on the periodic table, and the atomic weight is located directly under the element name. Round the atomic weight to the nearest whole number. Gold has an atomic number of 79 and an atomic weight of 196.966569, or 197.
Step 3
Calculate the number of neutrons by subtracting the atomic number from the atomic weight. The atomic number is equal to the number of protons in an atom. The atomic weight is equal to the total number of particles in the atom's nucleus. Since protons and neutrons occupy the nucleus together, subtracting the number of protons from the total particles will give you the number of neutrons. (For gold: 197 – 79 = 118 neutrons)
Things Needed
- Periodic table
- Calculator
TL;DR (Too Long; Didn't Read)
You can use the periodic table to find out the number of each particle in every type of element. The atomic number is not only the number of protons, but also the number of electrons as well.
Cite This Article
MLA
Cultrona, R.L.. "How To Find The Neutrons In The Periodic Table" sciencing.com, https://www.sciencing.com/neutrons-periodic-table-5845408/. 24 April 2017.
APA
Cultrona, R.L.. (2017, April 24). How To Find The Neutrons In The Periodic Table. sciencing.com. Retrieved from https://www.sciencing.com/neutrons-periodic-table-5845408/
Chicago
Cultrona, R.L.. How To Find The Neutrons In The Periodic Table last modified March 24, 2022. https://www.sciencing.com/neutrons-periodic-table-5845408/