How To Make An Atom Arsenic Model
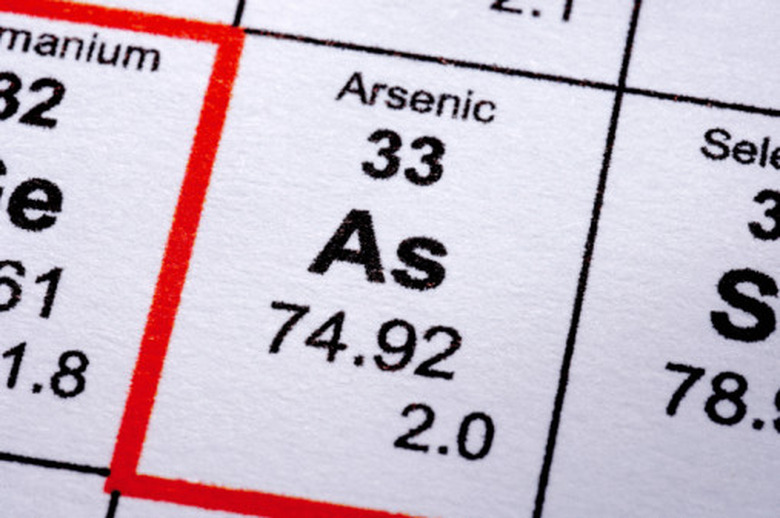
Arsenic is the 33rd element on the periodic table. It is most well-known in liquid or powder form, in which it was once used to kill mice and other pests and is still sometimes used as a poison. Because arsenic is so lethal, many people are surprised to find out that it is, in fact, a natural substance found commonly in the crust of the Earth. Because of its infamy, arsenic is the element to choose if you must give a chemistry presentation. You can construct a model atom of arsenic from everyday materials to use as a visual aid.
Model an Arsenic Atom
Step 1
Spray paint 33 of the foam balls bright red and the other 42 blue; the red balls will represent protons and the blue balls will represent neutrons. Glue all of the proton balls together using a cool-melt glue gun and let them dry. Glue the neutrons randomly to the outside of the proton cluster and let these dry, as well.
Step 2
Stick a 2-inch piece of plain floral wire into each side of the nucleus you made in step 1. Make sure the wires are straight across from each other. Attach one end of a pipe cleaner to each of the wires and form the pipe cleaner into an arch, then do the same with a second pipe cleaner on the opposite side of the nucleus.
Step 3
Look at your atom; you should see the foam nucleus with a circle of pipe cleaner around it held by the nearly invisible floral wire. Attach two more pieces of floral wire to opposite sides of the pipe cleaner ring, which represents an electron field, and create another pipe cleaner circle around it.
Step 4
Continue making rings from floral wire and pipe cleaners until you have four rings; twist together the ends of multiple pipe cleaners when necessary to make them longer. Attach two pom-poms very close together on the ring nearest to the nucleus with your cool-melt glue gun. Glue eight pom-poms onto the second ring and 18 to the third ring, placing them in pairs of two.
Step 5
Glue five pom-poms to the outside ring of pipe cleaners. Make two pairs and affix them across from each other, then place the lone pom-pom electron somewhere in-between. Let the glue dry on the electrons and your arsenic atom is complete.
Things Needed
- Red spray paint
- Blue spray paint
- 75 foam balls
- Cool-melt glue gun
- Cool-melt glue sticks
- 8 floral wires, 2 inches long
- Gray pipe cleaners
- 33 small white pom-poms
References
- Chemical Elements.com – Arsenic (As)
- Jefferson Lab; Questions and Answers; How Do I Make a Model of an Atom?
- WebElements Periodic Table of the Elements; Arsenic; Essential Information
- Q&A: How to Build a Model Atom; Department of Physics; University of Illinois at Urbana-Champaign
- GreenFacts: Scientific Facts on Arsenic
Cite This Article
MLA
Townsend, Jourdan. "How To Make An Atom Arsenic Model" sciencing.com, https://www.sciencing.com/make-atom-arsenic-model-8494104/. 24 April 2017.
APA
Townsend, Jourdan. (2017, April 24). How To Make An Atom Arsenic Model. sciencing.com. Retrieved from https://www.sciencing.com/make-atom-arsenic-model-8494104/
Chicago
Townsend, Jourdan. How To Make An Atom Arsenic Model last modified March 24, 2022. https://www.sciencing.com/make-atom-arsenic-model-8494104/