What Are Subscripts In A Chemical Formula Used To Indicate?
Though a simple component of any basic chemistry course, chemical formulas provide vital information about ions and compounds, and subscripts are just as important as the elements themselves. For example, the subscript and what it represents is what distinguishes the toxic gas carbon monoxide (CO) from carbon dioxide (CO2), a gas formed in human respiration and consumed in photosynthesis.
TL;DR (Too Long; Didn't Read)
Subscript numbers tell you how many of each element, chemical group or ion are present in a molecule.
Chemical Formulas
Chemical Formulas
Chemical formulas use letters and numbers to represent chemical species (i.e., compounds, ions).
The letters come from the periodic table and represent elements present in the species. An element may be represented by one capital letter, or one capital and one lowercase letter. (In rare cases, one capital letter and two lowercase letters may be used.) The letter or letters that represent an element are called its atomic symbol.
The numbers appearing as subscripts in the chemical formula indicate the number of atoms of the element immediately before the subscript. If no subscript appears, one atom of that element is present.
Chemical Structure
Chemical Structure
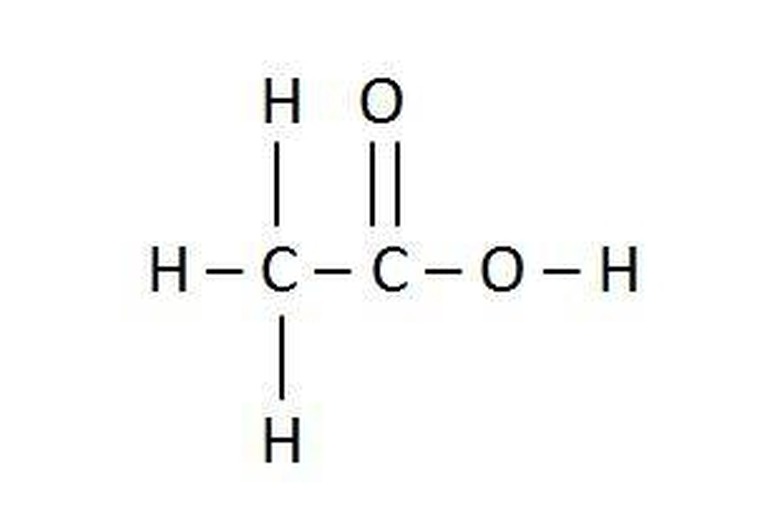
Subscripts in chemical formulas can also indicate the structure of the species, especially organic species.
Note the organic molecule acetic acid given in the picture. The chemical formula C2H4O2 is correct, but the formula CH3COOH gives chemists a clearer picture of the molecule.
Subscripts and Parentheses
Subscripts and Parentheses
Sometimes, chemists want to quickly recognize ions or chemical species present in a compound. When such a species appears more than once in a compound, it's common to enclose the species in parentheses. The subscript immediately following the end parenthesis indicates how many times that species appears in the compound.
For example, the formula Ca(NO3)2 indicates that two NO3- (nitrate) ions are present in the compound Ca(NO3)2.
Stoichiometry
Stoichiometry
Stoichiometry is the process of balancing chemical equations, and subscripts in chemical formulas play in important role. Chemistry students use subscripts to calculate the number of moles (a measurement of the amount of a substance) of each element on each side of the equation.
It's important to remember that each subscript is an unchangeable part of a compound's identity. When balancing equations, only the coefficients (the numbers in front of compounds in a chemical equation) are changed, not the subscripts.
Polymers
Polymers
A polymer is a large compound in which a group of elements appears several times in a row. That group of elements is called a monomer. In a chemical formula, monomers appear in parentheses, just like ions, only in the middle of the formula. The subscript belonging to a monomer doesn't need to be a number; it can be a variable.
For example, a monomer in polypropylene can be represented as (CH2CHCH3)n.
References
Cite This Article
MLA
Boley, Allison. "What Are Subscripts In A Chemical Formula Used To Indicate?" sciencing.com, https://www.sciencing.com/subscripts-chemical-formula-used-indicate-2461/. 30 April 2018.
APA
Boley, Allison. (2018, April 30). What Are Subscripts In A Chemical Formula Used To Indicate?. sciencing.com. Retrieved from https://www.sciencing.com/subscripts-chemical-formula-used-indicate-2461/
Chicago
Boley, Allison. What Are Subscripts In A Chemical Formula Used To Indicate? last modified August 30, 2022. https://www.sciencing.com/subscripts-chemical-formula-used-indicate-2461/