Every Northern And Southern Lights Color Explained
The auroras — the northern and southern lights — are undoubtedly one of the most spectacular natural displays in the world. If you're in the right place at the right time to see the northern or southern lights, you'll be witness to shimmering curtains of color dancing above you in the night sky. On a normal night, you'll probably just see green lights billowing in the sky, but occasionally the auroras put on a show with red or blue lights. And if you're really lucky, you might see yellow or pink.
To understand how these different colors are made, it helps to understand the basic mechanism of how the auroras are produced. It all starts at the sun. Besides light and heat, the sun is emitting a constant stream of high-energy particles (mostly hydrogen and helium nuclei stripped of their electron shells) called the solar wind. Most of this stream that reaches Earth is deflected off of the magnetosphere, but some is funneled towards the poles where it interacts with the atmosphere.
It's this interaction between high-energy particles and the atmosphere that creates the brilliant light of an aurora. When the particles collide with nitrogen and oxygen in the atmosphere, they transfer a bit of their energy to the atmospheric atoms, which pops their electrons up an energy level. When those electrons inevitably come back to their ground state, the atoms each emit a photon of light, which en masse creates what we see as an aurora.
Why are the northern and southern lights green?
To the naked eye, the most common color of the auroras is green, partly because human eyes are fine-tuned to see the color green, making it easier to distinguish in low-light scenarios. The main source of green light in the auroras is atomic oxygen (in contrast to the molecular oxygen you normally breathe). This light can only be produced above a certain altitude (typically around 60 miles up). This is because when atomic oxygen is excited by a high-energy particle, it doesn't shed that energy right away. In contrast with a gas like sodium or neon, which returns to its ground state in a millionth of a second, atomic oxygen takes about three-quarters of a second to calm down from being excited.
If, during that time, the excited oxygen atom has a collision or chemical reaction with other elements, it could shed that energy before it has a chance to release it as a photon. This happens at lower altitudes because the increasing density of the atmosphere creates more opportunities for these interactions to happen, robbing the oxygen of the time it needs to glow green. This is also why the green in auroras tends to have a distinct lower bound.
How do red auroras form?
Red auroras can form both above and below the most common green bands, and the source of the red color depends on whether it is above or below the green. Above the green bands (over 150 miles up), the source of red emission is again from oxygen. Up here, collisions with other atmospheric particles aren't as common as deeper in the atmosphere, so oxygen has more time to "recover" after emitting a green photon. There's still energy tied up in the excited electrons of these oxygen atoms, and as long as they don't collide with other atmospheric atoms or molecules, after a couple of minutes they can emit another burst of red light and finally return to their ground state.
The red you may see just below the green auroras comes from molecular nitrogen and is slightly violet or purple. This red lower fringe to auroras is rare because only the most energetic of solar particles can penetrate below 60 miles, where nitrogen is more abundant than oxygen.
Occasionally, during intense solar storms, red auroras can be seen far beyond the polar circles where most auroras are usually observed. This happens when there is a large burst of solar particles, like a coronal mass ejection, that crashes into oxygen atoms 200 miles up in the atmosphere. The sheer volume of high-energy particles ensures that lots of light will be emitted, and the fact that it's happening 200 miles up ensures that it can be seen from far away.
What causes blue, purple, pink, and yellow auroras?
The blue or purple light of an aurora can come from three different molecular sources, the most prominent of which is ionized molecular nitrogen. Ionized nitrogen most often glows blue around 60 miles up and is only visible during intense solar activity. Because ionized and non-ionized nitrogen occurs in the same general area, their colors can mix, producing colors from red and magenta to blue and purple.
Blues and purples can also be produced by hydrogen and helium high in the atmosphere (over 180 miles). Due to the scarcity of gas at this altitude, these colors are hard to see and typically only show up under dark skies and intense solar activity.
Yellow auroras arise from the same color mixing as pink or magenta auroras, only in this case, it's the red glow from non-ionized molecular nitrogen mixing with the green of oxygen. This is rare for two reasons: The first is the unique solar weather required to trigger low-altitude nitrogen emissions, the other is the mixing of that low-altitude nitrogen with the slightly higher oxygen.
What color are auroras on other planets?
Earth isn't the only place in the solar system with auroras. In fact, auroras have been observed around every planet except Mercury, which has no atmosphere for the sun's wind to interact with. Despite having no planetary magnetosphere, auroras have been observed on both Venus and Mars. While Earth's magnetosphere directs and concentrates incoming solar particles to the polar regions, on Venus and Mars the auroras can happen anywhere bombarded by sufficient solar particles.
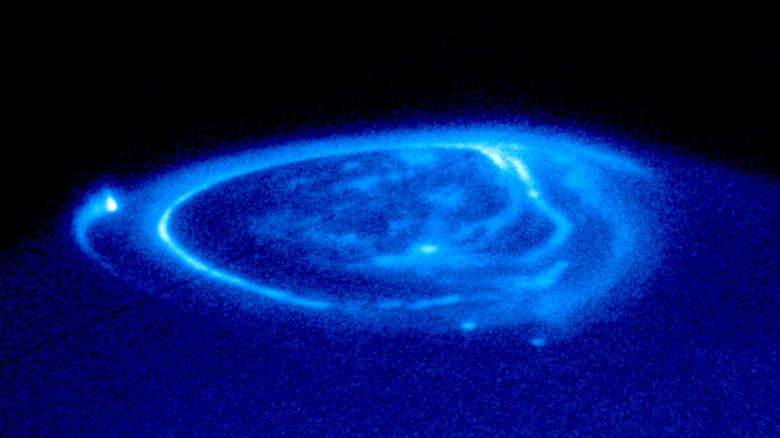
All of the gas giant planets have auroras that emit ultraviolet light. Jupiter's auroras are so intense that they actually emit X-rays! Saturn's auroras emit some visible light and would be red if you were to fly by, while Uranus also has auroras in the infrared spectrum, and Neptune has auroras that emit radio waves.
Beyond the planets, five moons have been found to have auroras as well. The four Galilean moons of Jupiter have visible auroras, all of which include red oxygen-based light, but Io also has orange emissions from atmospheric sodium. Neptune's moon Triton also appears to have an aurora, but due to how far away it is, little is known about it.