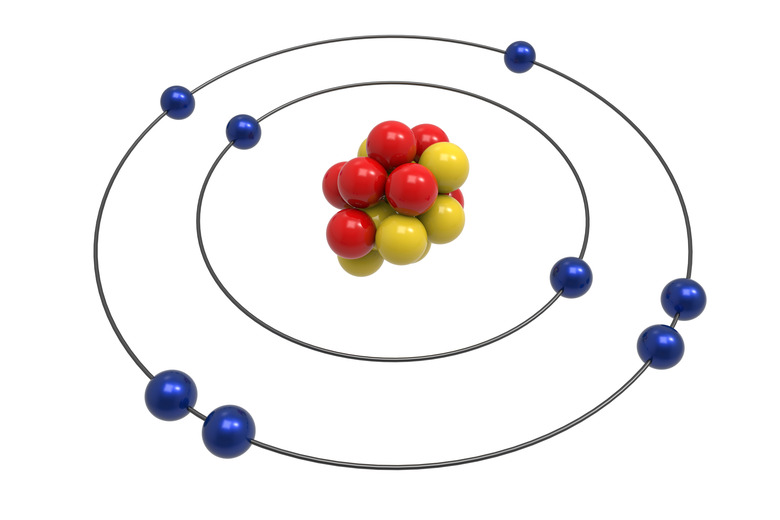
What Are The 4 Atomic Models?
The atom is the most basic unit of any element that still maintains the properties of that element. Because atoms are far too small to see, their structure has always been something of a mystery. For thousands of years, philosophers and scientists have proposed theories concerning the make-up of this mysterious particle, with increasing degrees of sophistication. Although there were many models, four main ones have led to our current concept of the atom.
The Plum Pudding Model
The Plum Pudding Model
The so-called plum pudding model was proposed by the scientist J.J. Thomson in 1904. This model was conceived after Thomson's discovery of the electron as a discrete particle, but before it was understood that the atom had a central nucleus. In this model, the atom is a ball of positive charge — the pudding — in which the electrons — the plums — are located. The electrons rotate in defined circular paths within the positive blob that makes up the majority of the atom.
Planetary Model
Planetary Model
This theory was proposed by the Nobel Prize winning chemist Ernest Rutherford in 1911 and is sometimes called the Rutherford model. Based on experiments that showed the atom appeared to contain a small core of positive charge, Rutherford postulated that the atom consisted of a small, dense and positively charged nucleus, around which electrons orbited in circular rings. This model was one of the first to propose the odd idea that atoms are mostly made up of empty space through which the electrons move.
Bohr Model
Bohr Model
The Bohr model was devised by Neils Bohr, a physicist from Denmark who received the Nobel prize for his work on the atom. In some ways it is a more sophisticated enhancement of the Rutherford model. Bohr proposed, as did Rutherford, that the atom had a small, positive nucleus where most of its mass resided. He stated that the electrons orbited around this nucleus like planets around the sun. The main improvement of Bohr's model was that the electrons were confined to set orbits around the nucleus, each having a specific energy level, which explained experimental observations such as electromagnetic radiation.
Electron Cloud Model
Electron Cloud Model
The electron cloud model is currently the most sophisticated and widely accepted model of the atom. It retains the concept of the nucleus from Bohr and Rutherford's models, but introduces a different definition of the motion of electrons around the nucleus. The movement of electrons around the nucleus in this model is defined by regions where there is a greater probability of finding the electron at any given moment. These regions of probability around the nucleus are associated with specific energy levels and take on a variety of odd shapes as the energy of the electrons increase.
Cite This Article
MLA
Judge, Michael. "What Are The 4 Atomic Models?" sciencing.com, https://www.sciencing.com/4-atomic-models-8121716/. 13 March 2018.
APA
Judge, Michael. (2018, March 13). What Are The 4 Atomic Models?. sciencing.com. Retrieved from https://www.sciencing.com/4-atomic-models-8121716/
Chicago
Judge, Michael. What Are The 4 Atomic Models? last modified March 24, 2022. https://www.sciencing.com/4-atomic-models-8121716/