Atomic Number Vs. Atomic Density
Atomic density means the number of atoms per unit volume. The atomic number of an element represents the number of protons in the nucleus and the number of electrons surrounding it.
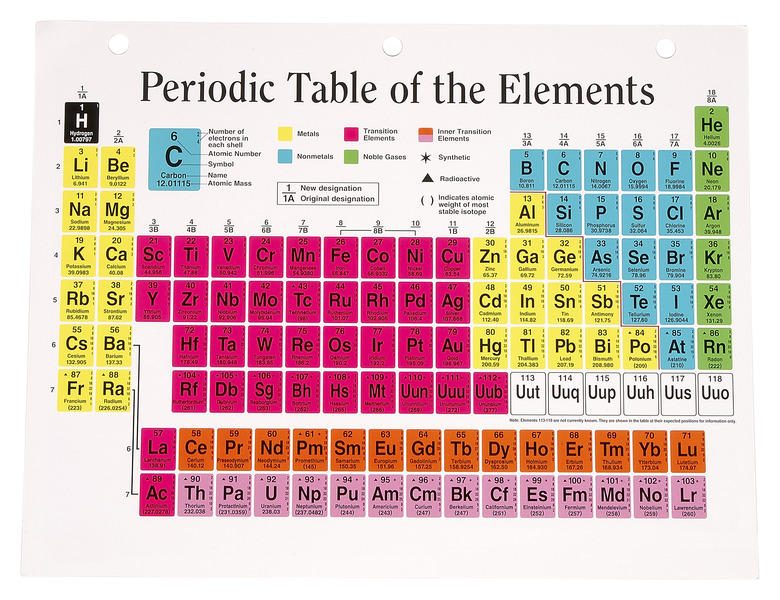
Periodic table of the elements
Periodic table of the elements
The periodic table of elements lists elements in ascending order according to its atomic number. Hydrogen, the first element in the periodic table, has the atomic number of one, corresponding with the number of protons in the nucleus. The last element in the periodic table is Ununoctium, and it has 118 protons in the nucleus.
Atomic weight
Atomic weight
As the atomic number increase, the actual weight of each of the elements also tends to increase. When looking at the periodic table, the elements generally get heavier going down each column and to the right across each row.
Density
Density
The density of an atom is determined by the number of atoms a substance per unit volume of the material. Solids will have a higher atomic density than gases.
Atomic number
Atomic number
The atomic number refers to the number of protons in the nucleus of a substance. Since undisturbed elements have a neutral electrical charge, the number of electrons is also the same as the atomic number. Except for a few cases, the atomic weight increases as does the atomic number.
Synthetic elements
Synthetic elements
Elements with the atomic numbers 104-118 are synthetically-produced materials that have not been found in the natural world. They have only been produced in places such as laboratories or particle accelerators. Ununoctium was discovered as recently as 2002 in Dubna, Russia.
Cite This Article
MLA
Contributor, . "Atomic Number Vs. Atomic Density" sciencing.com, https://www.sciencing.com/atomic-number-vs-atomic-density-5746698/. 24 April 2017.
APA
Contributor, . (2017, April 24). Atomic Number Vs. Atomic Density. sciencing.com. Retrieved from https://www.sciencing.com/atomic-number-vs-atomic-density-5746698/
Chicago
Contributor, . Atomic Number Vs. Atomic Density last modified March 24, 2022. https://www.sciencing.com/atomic-number-vs-atomic-density-5746698/