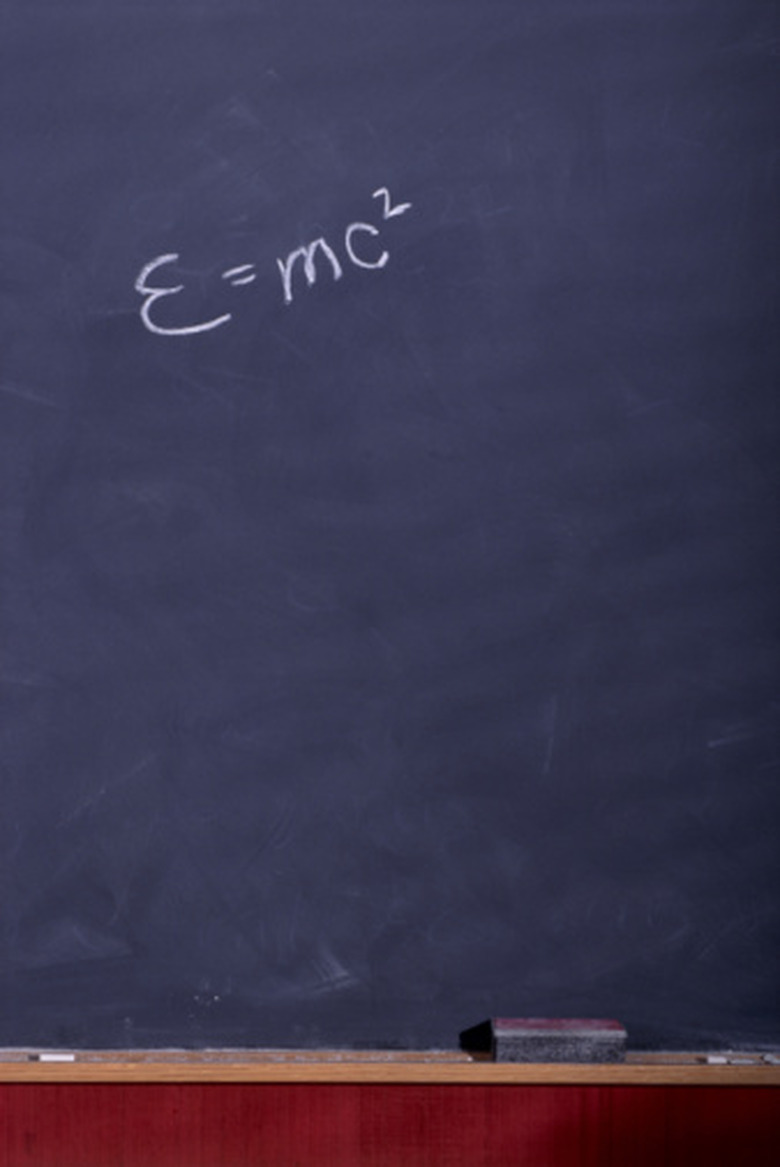How To Calculate The Momentum Of A Photon Of Yellow Light In A Wavelength
Photons exhibit what's known as "wave-particle duality," meaning that in some ways light behaves as a wave (in that it refracts and can be superimposed on other light) and in other ways as a particle (in that it carries and can transfer momentum). Even though a photon has no mass (a property of waves), early physicists found that photons hitting metal could displace electrons (a property of particles) in what's known as the photoelectric effect.
Step 1
Determine the light's frequency from its wavelength. The frequency (f) and wavelength (d) are related by the equation f = c/d, where c is the speed of light (approximately 2.99 x 10^8 meters per second). A specific yellow light might be 570 nanometers in wavelength, therefore, (2.99 x 10^8)/(570 x 10^-9) = 5.24 x 10^14. The yellow light's frequency is 5.24 x 10^14 Hertz.
Step 2
Determine the light's energy using Planck's constant (h) and the particle's frequency. The energy (E) of a photon is related to Planck's constant and the photon's frequency (f) by the equation E = hf. Planck's constant is approximately 6.626 x 10^-34 m^2 kilograms per second. In the example, (6.626 x 10^-34) x (5.24 x 10^14) = 3.47 x 10^-19. The energy of this yellow light is 3.47 x 10^-19 Joules.
Step 3
Divide the photon's energy by the speed of light. In the example, (3.47 x 10^-19)/(2.99 x 10^8 ) = 1.16 x 10^-27. The momentum of the photon is 1.16 x 10^-27 kilogram meters per second.
Cite This Article
MLA
Lecocq, Dan. "How To Calculate The Momentum Of A Photon Of Yellow Light In A Wavelength" sciencing.com, https://www.sciencing.com/calculate-photon-yellow-light-wavelength-8146440/. 24 April 2017.
APA
Lecocq, Dan. (2017, April 24). How To Calculate The Momentum Of A Photon Of Yellow Light In A Wavelength. sciencing.com. Retrieved from https://www.sciencing.com/calculate-photon-yellow-light-wavelength-8146440/
Chicago
Lecocq, Dan. How To Calculate The Momentum Of A Photon Of Yellow Light In A Wavelength last modified March 24, 2022. https://www.sciencing.com/calculate-photon-yellow-light-wavelength-8146440/