How To Calculate Valency
Valency is a measure of the ability of an atom to bond with other atoms. The higher the number of valent electrons, the more reactive the atom or molecule is.
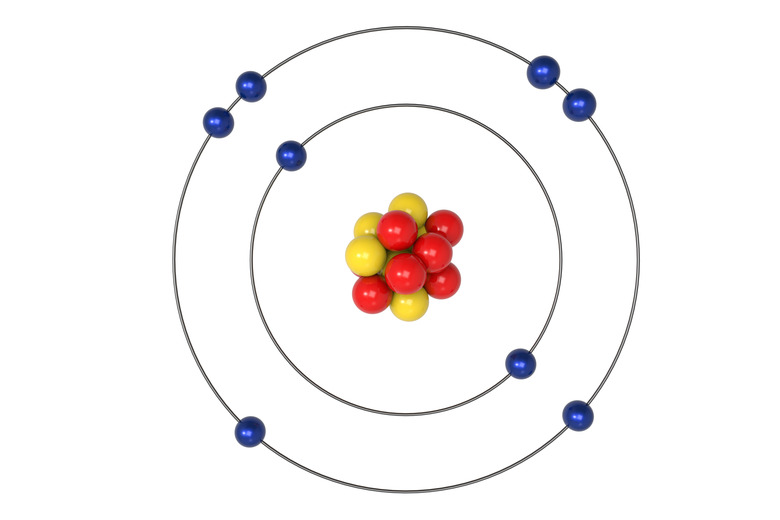
How Many Electrons in Each Orbital?
How Many Electrons in Each Orbital?
Electrons will occupy the most stable position first. The inner orbital (K) holds up to 2 electrons. The next orbital (L) holds up to 8 electrons. The next orbital (M) also holds up to 8 electrons.
There are s, p, d and f sub-orbitals that are within the K, L, M, N orbitals.
The presence of 8 electrons in the L orbital provides stability based on the orbital being full. By having 2 in the s sub-orbital and 2 in each of the 3 p sub-orbitals, this makes the L orbital complete. This applies to the M orbital as well. This is referred to as the Octet Rule.
Find the Valency Number
Find the Valency Number
Use the periodic table to find the atomic number. For the first example, let's use carbon. The atomic number is 6, which means 6 protons and 6 electrons.
The inner orbital of electrons has 2 electrons, so the next orbital has 4 (6 – 2 = 4).
The outer orbital, with 4 electrons moving in various ways, circling the nucleus, can create 4 single bonds.
You would say that the valency of carbon is 4.
Periodic Table Groups
Periodic Table Groups
The periodic table arranges the elements in a specific pattern based on their behavior. Elements with the same number of valence electrons have the same properties. The groups are named by the element at the top of the column on the periodic table.
The Group 1A Lithium Family has 1 valence electron. The atoms in this column of the periodic table tend to lose 1 electron, and this bonds it to an atom that prefers to accept 1 electron.
Elements in the Beryllium group have 2 valence electrons, and elements in the Oxygen group have 6. In keeping with the pattern of elements wanting to have a full shell of electrons, oxygen group elements like to gain 2 electrons.
The Helium Family, also called the Noble Gases, are non-reactive because they have no openings in their outer electron shell.
The valency of elements such as iron that are in the metals family is more complex and can have different valencies, depending on the forces from the other atoms around it. Some may have a valency of +2 under some circumstances and +3 under others. One of the reasons for this variance is that in larger molecules the orbitals are farther from the nucleus, which means that the force that keeps an electron with the atom is weaker. Another reason is that the orbitals are sometimes close to each other or overlap.
Valency of Boron (B)
Valency of Boron (B)
The inner orbital is nearest to the center of the boron atom and contains 2 electrons.
The next orbital contains 3 electrons, divided into subshells s and p. There are 2 electrons in s and 1 electron in p. These are the outermost 3, so these are the reactive electrons. Each will bond with other atoms by sharing an electron.
The valency of boron is 3.
Predicting Electron Behavior
Predicting Electron Behavior
Electrons fill up atomic orbitals in a particular pattern. The aufbau principle states that electrons are present in atomic orbitals beginning with the lowest-energy electrons, followed by successively higher-energy electrons.
Orbital 1s fills before 2s, which fills before 2p and so on. Each s, p and d orbital has a capacity for 2 electrons, which spin in opposite directions.
Valency is important to know because it allows you to predict if the atom is more likely to **donate **electrons or **accept **them, and this allows you to know how the atom will interact with other atoms.
Cite This Article
MLA
McConnell, Tracy. "How To Calculate Valency" sciencing.com, https://www.sciencing.com/calculate-valency-2790/. 10 February 2020.
APA
McConnell, Tracy. (2020, February 10). How To Calculate Valency. sciencing.com. Retrieved from https://www.sciencing.com/calculate-valency-2790/
Chicago
McConnell, Tracy. How To Calculate Valency last modified March 24, 2022. https://www.sciencing.com/calculate-valency-2790/