How To Calculate X-Ray Energy
The general formula for energy of a single photon of an electromagnetic wave such as an X-ray is given by Planck's equation:
\(E=h\nu\)
in which energy E in Joules is equal to the the product of Planck's constant h (6.626 × 10 −34 Js) and the frequency ν (pronounced "nu") in units of s_-1_. For a given frequency of an electromagnetic wave, you can calculate the associated X-ray energy for a single photon using this equation. It applies to all forms of electromagnetic radiation including visible light, gamma rays, and X-rays.
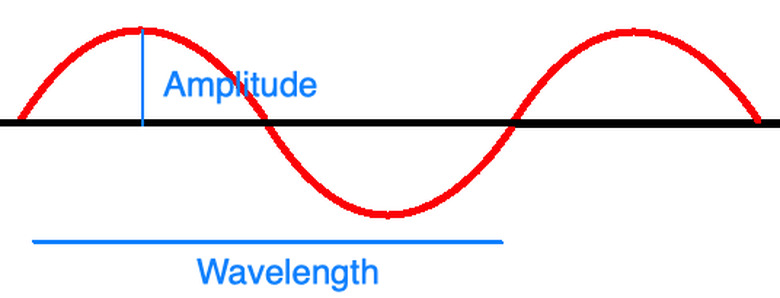
Planck's equation depends on wavelike properties of light. If you imagine light as a wave as shown in the diagram above, you can imagine it having an amplitude, frequency, and wavelength just as an ocean wave or a sound wave might. The amplitude measures the height of one crest as shown and generally corresponds to the brightness or intensity of the wave, and the wavelength measures the horizontal distance that a full cycle of the wave covers. The frequency is the number of full wavelengths that pass by a given point every second.
X-rays as Waves
X-rays as Waves
As part of the electromagnetic spectrum, you can determine either the frequency or wavelength of an X-ray when you know one or the other. Similar to Planck's equation, this frequency ν of an electromagnetic wave relates to the speed of light c, 3 x 10-8 m/s, with the equation
\(c=\lambda \nu\)
in which λ is the wavelength of the wave. The speed of light remains constant in all situations and examples, so this equation demonstrates how frequency and wavelength of an electromagnetic wave are inversely proportional to one another.
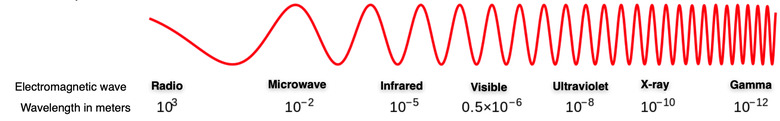
In the above diagram, the various wavelengths of different types of waves are shown. X-rays lie between ultraviolet (UV) and gamma rays in the spectrum so X-ray properties of wavelength and frequency fall between them.
Shorter wavelengths indicate greater energy and frequency that can pose risks to human health. Sunscreens that block against UV rays and protective coats and shields of lead that block X-rays from entering skin demonstrate this power. Gamma rays from outer space are fortunately absorbed by the Earth's atmosphere, preventing them from harming people.
Finally, frequency can be related to period T in seconds with the equation
\(T=\frac{1}{f}\)
These x-ray properties can also apply to other forms of electromagnetic radiation. X-ray radiation in particular shows these wavelike properties, but also particle-like ones.
X-rays as Particles
X-rays as Particles
In addition to wavelike behaviors, X-rays behave like a stream of particles as though a single wave of an X-ray consisted of one particle after another colliding with objects and, upon collision, absorb, reflect, or pass through.
Because Planck's equation uses energy in the form of single photons, scientists say electromagnetic waves of light are "quantized" into these "packets" of energy. They are made of specific amounts of photon that carry discrete amounts of energy called quanta. As atoms absorb or emit photons, they, respectively, increase in energy or lose it. This energy can take the form of electromagnetic radiation.
In 1923 American physicist William Duane explained how X-rays would diffract in crystals through these particle-like behaviors. Duane used the quantized momentum transfer from the geometric structure of the diffracting crystal to explain how different X-ray waves would behave when passing through the material.
X-rays, like other forms of electromagnetic radiation exhibit this wave-particle duality that lets scientists describe their behavior as though they were both particles and waves simultaneously. They flow like waves with a wavelength and frequency while emitting amounts of particles as though they were beams of particles.
Using X-ray Energy
Using X-ray Energy
Named after German physicist Maxwell Planck, Planck's equation dictates that light behaves in this wavelike manner, light also shows particle-like properties. This wave-particle duality of light means that, though the energy of light depends upon its frequency, it still comes in discrete amounts of energy dictated by photons.
When the photons of X-rays come into contact with different materials, some of them are absorbed by the material while others pass through. The X-rays that pass through let doctors create internal images of the human body.
X-rays in Practical Applications
X-rays in Practical Applications
Medicine, industry and various areas of research through physics and chemistry use X-rays in different ways. Medical imaging researchers use X-rays in creating diagnoses to treat conditions within the human body. Radiotherapy has applications in cancer treatment.
Industrial engineers use X-rays to ensure metals and other materials have the appropriate properties necessary for purposes such as identifying cracks in buildings or creating structures that can withstand large amounts of pressure.
Research on X-rays at synchrotron facilities lets companies manufacture scientific instruments used in spectroscopy and imaging. These synchrotrons use large magnets to bend light and force the photons to take wavelike trajectories When X-rays are accelerated in circular motions at these facilities, their radiation becomes linearly polarized to produce large amounts of power. The machine then redirects the X-rays towards other accelerators and facilities for research.
X-rays in Medicine
X-rays in Medicine
The applications of X-rays in medicine created entirely new, innovative methods of treatment. X-rays became integral to the process of identifying symptoms within the body through their non-invasive nature that would let them diagnose without the need to physically enter the body. X-rays also had the advantage of guiding physicians as they inserted, removed, or modified medical devices within patients.
There are three main types of X-rays imaging used in medicine. The first, radiography, images the skeletal system with only small amounts of radiation. The second, fluoroscopy, lets professionals view the internal state of a patient in real-time. Medical researchers have used this to feed patients barium to observe the workings of their digestive tract and diagnose esophageal diseases and disorders.
Finally, computed tomography lets patients lie down underneath a ring-shaped scanner to create a three-dimensional image of the patient's internal organs and structures. The three-dimensional images are aggregated together from many cross-sectional images taken of the patient's body.
X-ray History: Inception
X-ray History: Inception
German mechanical engineer Wilhelm Conrad Roentgen discovered X-rays while he was working with cathode-ray tubes, a device that fired electrons to produce images. The tube used a glass envelope that protected the electrodes in a vacuum inside the tube. By sending electrical currents through the tube, Roentgen observed how different electromagnetic waves were emitted from the device.
When Roentgen used a thick black paper to protect the tube, he found that the tube emitted a green fluorescent light, an X-ray, that could pass through the paper and energize other materials. He found that, when charged electrons of a certain amount of energy would collide with material, X-rays were produced.
Naming them "X-rays," Roentgen hoped to capture their mysterious, unknown nature. Roentgen discovered it could pass through human tissue, but not through bone nor metal. In late 1895, the engineer created an image of his wife's hand using the X-rays as well as an image of weights in a box, a notable feat in X-ray history.
X-ray History: Spread
X-ray History: Spread
Soon, scientists and engineers became allured by the X-ray's mysterious nature began exploring the possibilities for X-ray use. The roentgen (R) would become a now-defunct unit of measuring radiation exposure that would be defined as the amount of exposure necessary to make a single positive and negative unit of electrostatic charge for dry air.
Producing images of the internal skeletal and organ structures of humans and other creatures, surgeons and medical researchers created innovative techniques of understanding the human body or figuring out where bullets were located in wounded soldiers.
By 1896, scientists were already applying the techniques to figure out which types of matter X-rays could pass through. Unfortunately, the tubes that produce X-rays would break down under the large amounts of voltage needed for industrial purposes until the 1913 Coolidge tubes of American physicist-engineer William D. Coolidge used a tungsten filament for more accurate visualization in the newly born field of radiology. Coolidge's work would ground X-ray tubes firmly in physics research.
Industrial work took off with the production of lightbulbs, fluorescent lamps and vacuum tubes. Manufacturing plants produced radiographs, X-ray images, of steel tubes to verify their internal structures and composition. By the 1930s General Electric Company had produced one million X-ray generators for industrial radiography. The American Society of Mechanical Engineers began using of X-rays for fusing welded pressure vessels together.
X-ray Negative Health Effects
X-ray Negative Health Effects
Given how much energy X-rays pack with their short wavelengths and high frequencies, as society embraced X-rays in various fields and disciplines, the exposure to X-rays would cause individuals to experience eye irritation, organ failure and skin burns, sometimes even resulting in the loss of limbs and lives. These wavelengths of the electromagnetic spectrum could break chemical bonds that would cause mutations in DNA or changes in molecular structure or cellular function in living tissues.
More recent research on X-rays has shown that these mutations and chemical aberrations can cause cancer, and scientists estimate 0.4 % of cancers in the United States are caused by CT scans. As X-rays rose in popularity, researchers began recommending levels of X-ray dosage that were deemed safe.
As society embraced the power of X-rays, physicians, scientists and other professionals began expressing their concerns about the negative health effects of X-rays. As researchers observed how X-rays would pass through the body without paying close attention to how the waves specifically targeted areas of the body, they had little reason to believe X-rays could be dangerous.
X-ray Safety
X-ray Safety
Despite the negative implications of X-ray technologies on human health, their effects can be controlled and maintained to prevent unnecessary harm or risk. While cancer naturally affects 1 in 5 Americans, a CT scan generally raises the risk of cancer by .05 percent, and some researchers argue that low X-ray exposure may not even contribute to an individual's risk of cancer.
The human body even has built-in ways of repairing damage caused by low dosages of X-rays, according to a study in the American Journal of Clinical Oncology, suggesting that X-ray scans do not pose any significant risk at all.
Children are at greater risk of brain cancer and leukemia when exposed to X-rays. For this reason, when a child may require an X-ray scan, physicians and other professionals discuss the risks with guardians of the child's family to provide consent.
X-rays on DNA
X-rays on DNA
Exposure to high amounts of X-rays can result in vomiting, bleeding, fainting, loss of hair and loss of skin. They can cause mutations in DNA because they have just enough energy to break bonds between DNA molecules.
It's still difficult to determine if mutations in DNA as due to X-ray radiation or random mutations of DNA itself. Scientists can study the nature of mutations including their probability, etiology and frequency to determine whether the double-strand breaks in DNA were the result of X-ray radiation or the random mutations of DNA itself.
Cite This Article
MLA
Ather, S. Hussain. "How To Calculate X-Ray Energy" sciencing.com, https://www.sciencing.com/calculate-xray-energy-5091080/. 27 December 2020.
APA
Ather, S. Hussain. (2020, December 27). How To Calculate X-Ray Energy. sciencing.com. Retrieved from https://www.sciencing.com/calculate-xray-energy-5091080/
Chicago
Ather, S. Hussain. How To Calculate X-Ray Energy last modified August 30, 2022. https://www.sciencing.com/calculate-xray-energy-5091080/