What Are The Components Of The Atomic Structure?
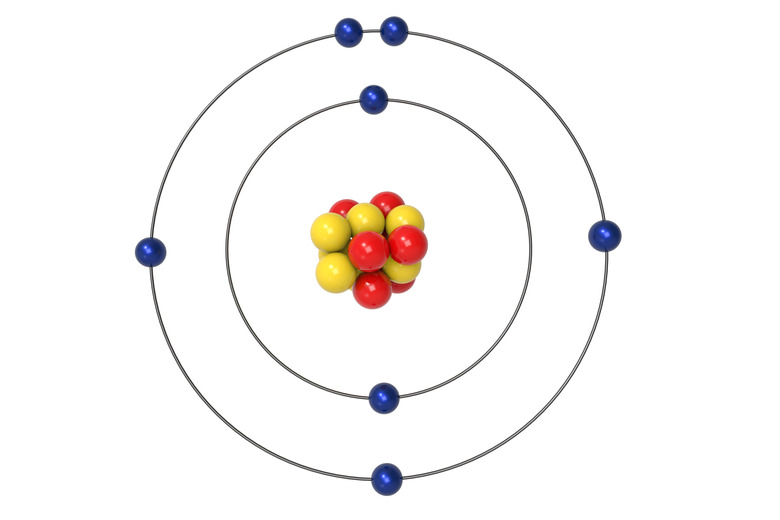
Atoms are the basic building blocks that comprise all matter in the universe. Each of the elements on the periodic table is composed of uniquely structured atoms. The elements are given different physical properties depending on their atomic building blocks. The atoms themselves are comprised of a different number of protons, neutrons and electrons, depending on the particular element. Each one of these separate sub-atomic particles has its own unique properties.
The Nucleus
The Nucleus
The nucleus of an atom contains the majority of the atom's mass, and is composed of protons and neutrons, which are collectively referred to as nucleons. The much-lighter electrons orbit their atom's nucleus. The number of protons and neutrons comprising an atom's nucleus determine that atom's mass number, sometimes referred to as "nucleon number."
The Protons
The Protons
Protons are positively charged particles found in an atom's nucleus. Along with neutrons, protons account for the vast majority of an atom's total mass. The total number of protons in an atom represents that atom's constant atomic number. Individual protons have a weight of 1.0073 on the carbon-12 scale, which is the scale that measures atoms' relative mass.
The Neutrons
The Neutrons
Neutrons are particles with a neutral charge which share the nucleus of an atom with protons. At 1.0087 on the carbon-12 scale, neutrons are so similar in weight to protons that the two particles are often considered to share the same general weight: a relative mass of 1. Where the number of protons in each element is a constant number, the number of neutrons can vary. For this reason, an element's mass number can vary from atom to atom.
The Electrons
The Electrons
Electrons are negatively charged particles that orbit the nucleus of an atom. These particles are much lighter than protons and neutrons, with a relative mass of 1/1836 the mass of protons. Electrons orbit the nucleus in a series of levels often referred to as "energy levels." Each of these levels can hold a specific number of electrons, the first level being closest to the nucleus, and subsequent levels being further and further away. The number of energy levels in an atom depends on the total number of electrons. Electrons will always settle into orbit at the lowest available level.
Cite This Article
MLA
Reynolds, Bill. "What Are The Components Of The Atomic Structure?" sciencing.com, https://www.sciencing.com/components-atomic-structure-14117/. 13 March 2018.
APA
Reynolds, Bill. (2018, March 13). What Are The Components Of The Atomic Structure?. sciencing.com. Retrieved from https://www.sciencing.com/components-atomic-structure-14117/
Chicago
Reynolds, Bill. What Are The Components Of The Atomic Structure? last modified March 24, 2022. https://www.sciencing.com/components-atomic-structure-14117/