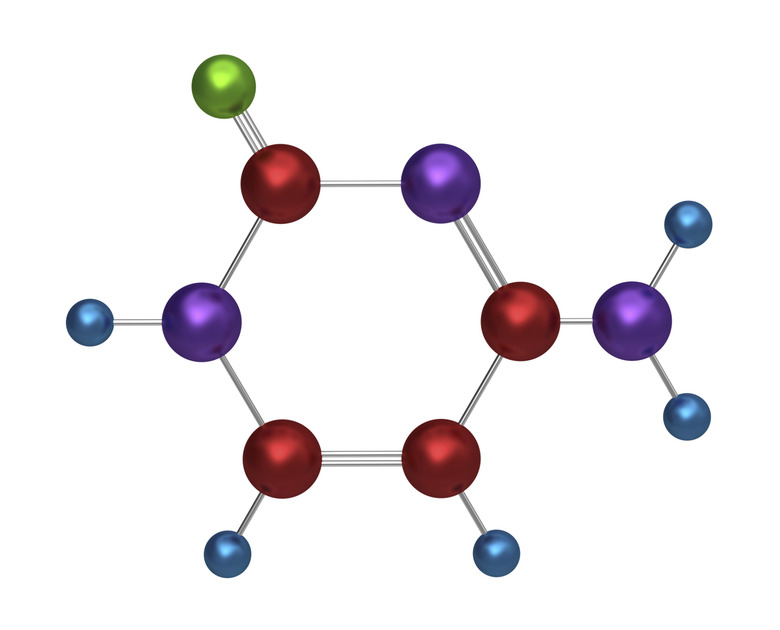
Covalent Vs. Hydrogen Bonds
Covalent bonds and hydrogen bonds are primary intermolecular forces. Covalent bonds can occur between most elements on the periodic table. Hydrogen bonds are a special bond between a hydrogen atom and an oxygen, nitrogen or fluorine atom.
Valence
Valence
The power of an element to combine with other elements is represented by an assigned number called the valence. For ions, the valence is equal to the electrical charge. For example, the valence for chlorine is 3p5, so it will easily gain one electron, and the resulting ion is Cl-.
The Octet Rule
The Octet Rule
The octet rule is based on the idea that the noble gas configuration (s2p6 ) is the most favorable and can be achieved with the formation of electron-pair bonds with other atoms.
Covalent Bonds
Covalent Bonds
Covalent bonds are formed when two or more atoms share electrons to fill their outermost electron shells.
Hydrogen Bonds
Hydrogen Bonds
A hydrogen bond occurs when the partial positive charge of a hydrogen atom bonds to an electronegative molecule, usually oxygen, nitrogen or fluorine.
Covalent v. Hydrogen Bonds
Covalent v. Hydrogen Bonds
Both covalent and hydrogen bonds are forms of intermolecular forces. Covalent bonds can occur with most elements on the periodic table, while hydrogen bonds usually occur between a hydrogen atom and an oxygen, nitrogen, or fluorine molecule. Also, hydrogen bonds are only about 1/10 as strong as a covalent bond.
References
Cite This Article
MLA
Gingrich, Jane. "Covalent Vs. Hydrogen Bonds" sciencing.com, https://www.sciencing.com/covalent-vs-hydrogen-bonds-5982030/. 24 April 2017.
APA
Gingrich, Jane. (2017, April 24). Covalent Vs. Hydrogen Bonds. sciencing.com. Retrieved from https://www.sciencing.com/covalent-vs-hydrogen-bonds-5982030/
Chicago
Gingrich, Jane. Covalent Vs. Hydrogen Bonds last modified March 24, 2022. https://www.sciencing.com/covalent-vs-hydrogen-bonds-5982030/