How To Determine Moles In Chemistry
In chemistry, a mole is a quantity used relate reactants to products in stoichiometric equations. A mole of any substance is equal to 6.02 x 10^23 particles — usually atoms or molecules — of that substance. For a given element, the mass (in grams) of one mole is given by its mass number on the periodic table; the "molar mass" of a molecule is the sum of the molar masses of the elements in the molecule in the correct ratios. It is simple to determine the molar mass of elements and molecules using the periodic table, as well as convert between grams and moles.
Determining the Molar Mass of an Element
Step 1
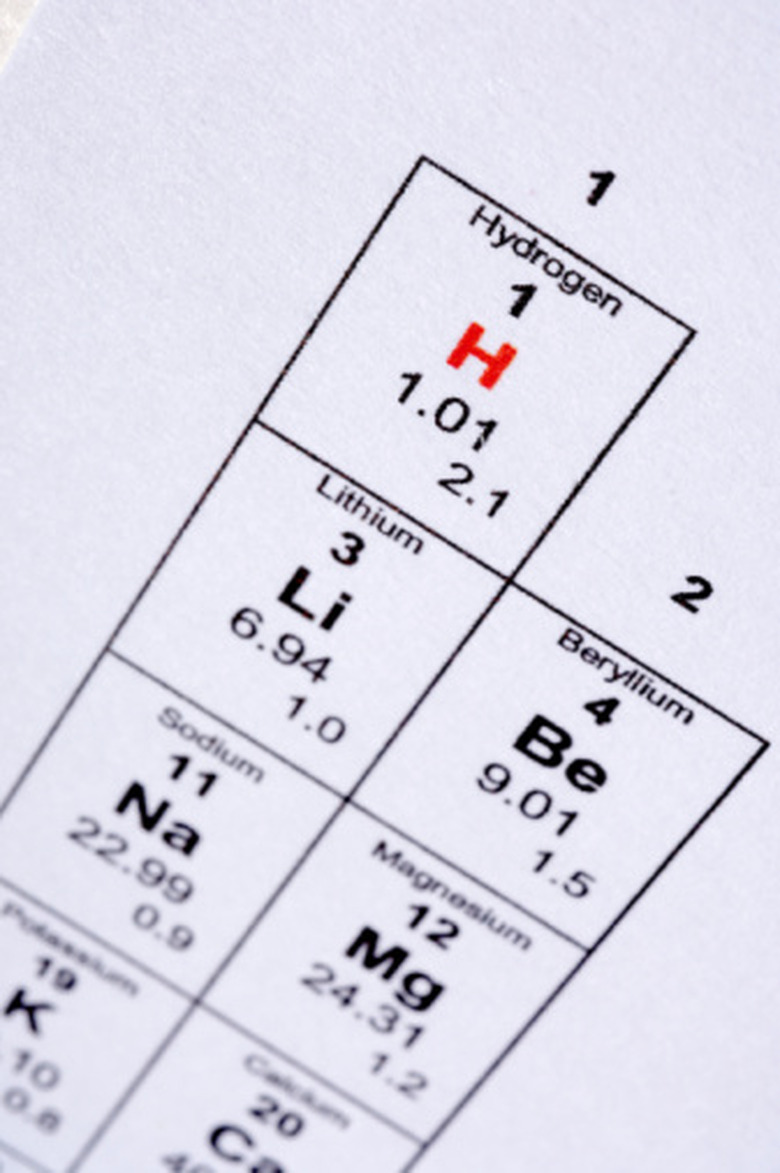
Find the element lithium (Li) on the periodic table. The atomic number for lithium is 3, representing the number of protons in the nucleus of one atom.
Step 2
Note that the mass number of lithium is 6.94, representing the sum of the numbers of protons and neutrons in the nucleus of one atom.
Step 3
Note that the mass number is equal to the mass (in grams) of one mole of lithium; this is the molar mass of lithium.
Determine the Molecular Mass of a Chemical Compound
Step 1
Determine the molecular mass of carbon dioxide (chemical formula CO2). Find carbon and oxygen on the periodic table.
Step 2
Note the masses of carbon and oxygen from the periodic table, which are 12.01 and 16, respectively.
Step 3
Add the mass numbers of one atom of carbon and two atoms of oxygen from the periodic table: 12.01 + 2(16) = 44.01 grams per mole
Converting From Mass to Moles
Step 1
Calculate the number of moles of water in 600 grams of water (H2O). Find hydrogen and oxygen on the periodic table.
Step 2
Set up the following equation relating grams to moles:
x moles H2O = (1 mole H2O/18 grams H2O) x (600 grams H2O)
Step 3
Solve the equation in Step 2 to find that there are 3.33 moles of H2O in 600 grams of H2O.
Cite This Article
MLA
Tyler, Judy. "How To Determine Moles In Chemistry" sciencing.com, https://www.sciencing.com/determine-moles-chemistry-8561700/. 24 April 2017.
APA
Tyler, Judy. (2017, April 24). How To Determine Moles In Chemistry. sciencing.com. Retrieved from https://www.sciencing.com/determine-moles-chemistry-8561700/
Chicago
Tyler, Judy. How To Determine Moles In Chemistry last modified August 30, 2022. https://www.sciencing.com/determine-moles-chemistry-8561700/