What Is The Difference Between Blue & Red Litmus Paper?
You've probably heard the term "litmus test" bandied about in the world of politics, especially when candidates are on the campaign trail. It references any question or predicament that can be used to make broader assumptions about someone's beliefs or how they might respond to future scenarios. This is only a metaphor, though. A real litmus test is something you're most likely to encounter in a chemistry lab.
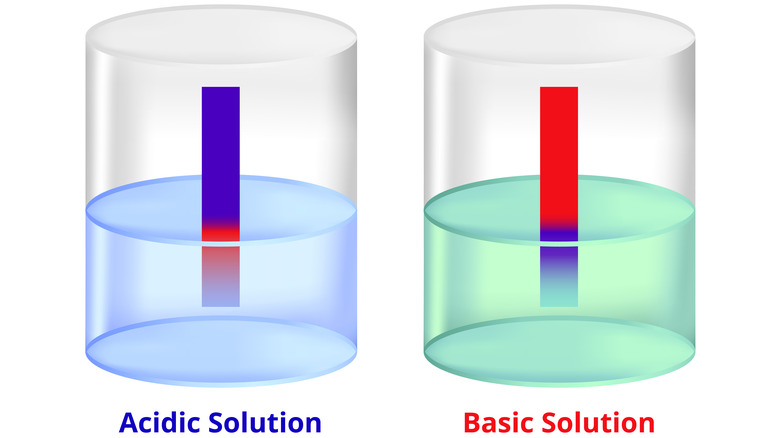
Litmus tests are used to determine whether a liquid is acidic or alkaline, that is, whether it has the characteristics of an acid or base. These tests are done by dipping strips of colored paper called litmus paper into the liquid in question and observing how the paper changes color. There are two basic types of litmus paper: red litmus paper and blue litmus paper.
Blue litmus paper turns red when it comes into contact with an acidic liquid with a pH level below 4.5, and stays blue when it comes into contact with a base. For red litmus paper, it's the exact opposite. Red litmus paper turns blue when it comes into contact with an alkaline (basic) liquid with a pH over 8.3, but stays red if it comes into contact with an acid. Either type of litmus paper can be used to test any substance, just so long as you remember that red means acidic and blue means basic.
How red and blue litmus papers are made
Both red and blue litmus papers are made by treating strips of ordinary paper with a series of dyes. The final product typically contains a combination of 10 to 15 different dyes. These dyes are derived from one of the most fascinating lifeforms on Earth: lichen. Often mistaken for moss, lichen can frequently be found on tree trunks. In fact, lichen has a symbiotic relationship with trees. Despite its appearance, lichen is not a plant, but is actually a union between fungus and algae. There are over 3,000 different lichen species in North America alone, but the main species used for litmus paper is called Rocella tinctoria.
The color-changing abilities of litmus dyes come from the chromophore 7-hydroxyphenoxazone. A chromophore is a part of a molecule that lends color by absorbing and reflecting different wavelengths of light. When 7-hydroxyphenoxazone comes into contact with an acid, it gains a proton, causing it to reflect red light. When it comes into contact with a base, it loses a proton, and reflects blue light.
Now here's the really interesting part. All litmus paper is naturally blue. However, as we know, acids turn blue litmus paper red. When you buy red litmus paper, you're really buying blue litmus paper that has been treated with an acid to turn it red. When the red litmus paper comes in contact with an alkaline solution, it returns to its natural blue state.
Litmus paper vs. universal pH indicators
Litmus papers are often confused with universal pH indicator strips, but the latter is actually a different — and arguably better — technology. Litmus paper has limits to its usefulness. While red litmus paper is effective at determining whether a substance is acidic, and vice-versa for blue litmus paper, neither type can tell you the actual pH level. The pH scale ranges from zero to 14, with seven being neutral. Every level lower than seven is acidic, and every level above is alkaline. However, red litmus paper is only effective at indicating acids with pH levels below 4.5 and blue litmus paper is only effective at indicating bases with pH levels above 8.3. Litmus paper can be useful for a broad overview, but if you want specifics, you need a universal indicator.
Universal indicator strips look just like litmus paper, but they are treated differently. Instead of the lichen-based dyes used for litmus paper, universal indicator solutions are made from synthetic dyes, including thymol blue, methyl red, bromothymol blue, and phenolphthalein. Universal indicator strips start out looking yellowish, but instead of changing to just two colors, they can react in a whole spectrum that covers the entire pH range. The colors vary by manufacturer, but the most common uses green to indicate a neutral pH level of seven, a spectrum of yellow to red indicating increasingly strong acids, and a spectrum of blue to purple indicating increasingly strong bases.