How Do Fruit Batteries Power An LED Light?
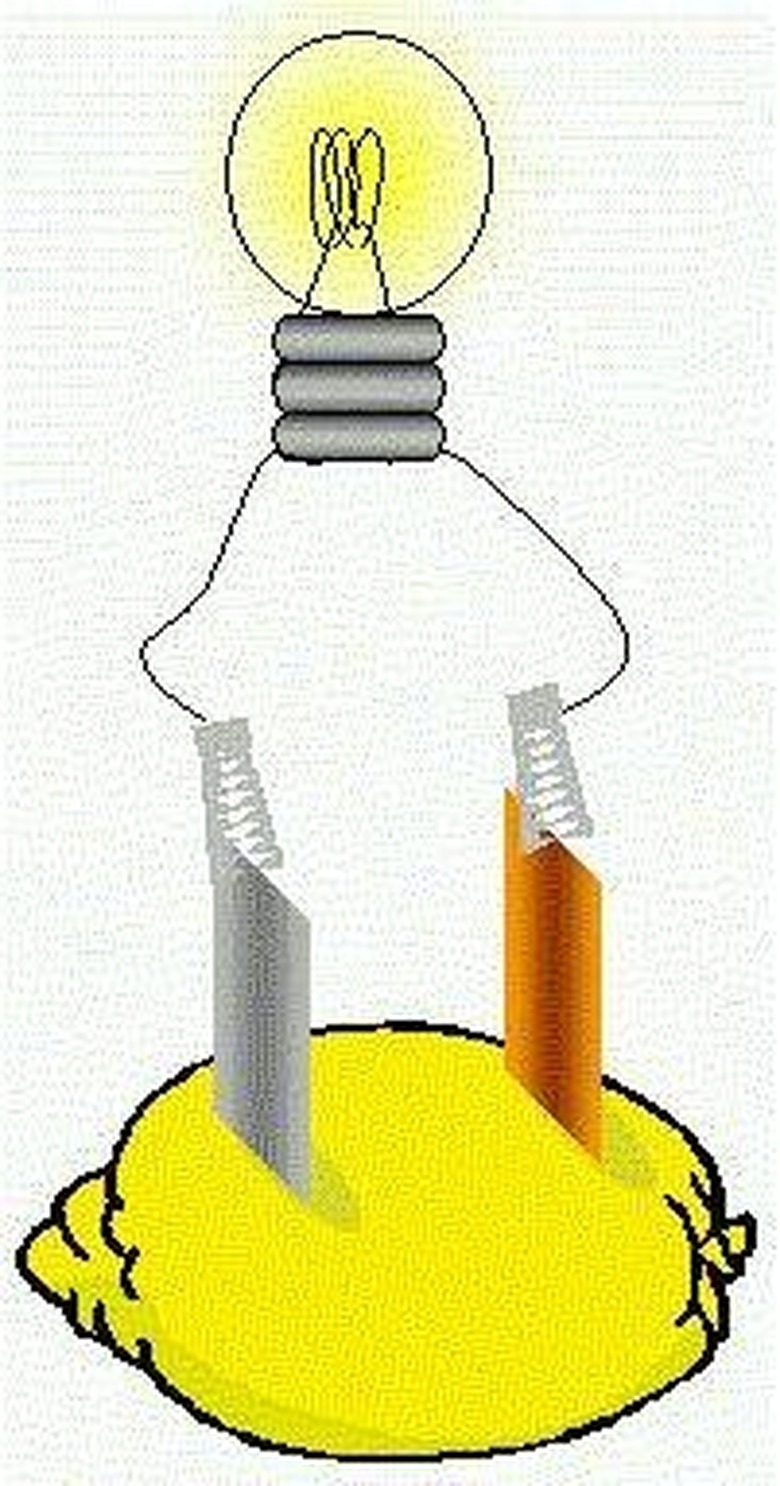
An acidic citrus fruit, such as a lemon or a lime, can be converted into a battery by inserting two 2-inch nails — one copper and one galvanized (zinc) — into the fruit. The amount of electrical current is small, but it's enough to power a light-emitting diode (LED).
Preparation
Preparation
Prepare the fruit for use as a battery by squeezing it gently, without breaking the skin, to release the juices inside it. Insert the nails into the fruit about two inches apart, making sure they do not touch each other to prevent shorting.
Function
Function
A reaction takes place between positively charged ions in the fruit and the zinc metal in the nail, liberating negatively charged particles, called electrons. Electrons travel from the positive pole, or terminal, of the battery through a copper wire — each end of which is connected to the nails with crocodile clips — to the negative pole. The movement of charge generates enough electricity to light the bulb.
LED
LED
An LED is often the bulb of choice in experiments of this kinds; it requires no more than 2.5 to 3 volts and a small current — in the order of thousandths of an amp, or milliamps (mA) — to operate.
References
Cite This Article
MLA
Dunning, David. "How Do Fruit Batteries Power An LED Light?" sciencing.com, https://www.sciencing.com/do-batteries-power-led-light-7725781/. 24 April 2017.
APA
Dunning, David. (2017, April 24). How Do Fruit Batteries Power An LED Light?. sciencing.com. Retrieved from https://www.sciencing.com/do-batteries-power-led-light-7725781/
Chicago
Dunning, David. How Do Fruit Batteries Power An LED Light? last modified March 24, 2022. https://www.sciencing.com/do-batteries-power-led-light-7725781/