Electromagnetic Spectrum: Frequencies, Wavelengths (W/ Diagrams & Examples)
Electromagnetic radiation (EM radiation) is all around you; it is fundamental not only to your understanding of physics, but also to your very survival. Without radiation from the sun, for example, Earth could not sustain life.
What scientists from NASA and their kin have come to call electromagnetic radiation can be described in physics terms as a wave. Electromagnetic waves are united by a few important characteristics, but their practical impact on the world varies from the ability to quickly cook food to transmitting information rapidly over enormous distances.
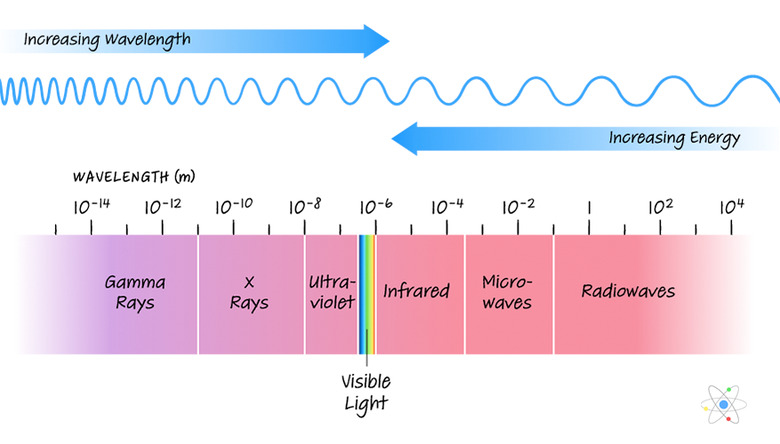
Shorter wavelengths are associated with higher frequencies and high energy, while higher frequencies sit on a short-wavelength portion of the spectrum with longer wavelengths. The relatively narrow visible spectrum offers the human eye, and thus the intuitive human mind, a far smaller picture of total reality than what's really going on all of the time.
What Are Electromagnetic Waves?
What Are Electromagnetic Waves?
An electromagnetic wave consists of an electric field wave oscillating in a plane perpendicular (at right angles) to a magnetic field wave. Since electromagnetic radiation acts as a wave, then any particular electromagnetic wave will have a frequency (f; sometimes denoted by ν instead) and wavelength λ (the Greek letter lambda) associated with it.
However, electromagnetic waves do not require a physical medium such as air (Earth's atmosphere is laden with gases and is not mere "space") or water through which to propagate, and hence can traverse the vacuum of empty space – which they do at the speed of light c, which is 3 × 108 m/s, or about 6 trillion miles an hour. Perhaps needless to say, this the fastest speed in the universe!
The Electromagnetic Spectrum
The Electromagnetic Spectrum
Electromagnetic waves can come in many different wavelengths and different frequencies, so long as the product of the wavelength and frequency of a given wave equals the speed of light (that is, λf = c). Think of a squad of wrestlers from the same narrow weight class; some will be taller and leaner, others shorter and more compact, but by definition all have very close to the same mass despite widely varying appearances.
The range of wavelengths across the EM spectrum is divided into a number of types of electromagnetic radiation. On order of lowest frequency (highest energy, longest wavelength) to highest frequency (lowest energy, shortest wavelength) these types of radiation are:
- **Radio waves:** These waves, which allowed for listening to "broadcasts" long before the Internet, have
λ values starting at about 1 m and extending beyond 108 m (100 million m).
- **Microwaves:** Microwave ovens are the most obvious technological manifestation of this kind of radiation, which spans the wavelength spectrum from about 10-3 m (1 millimeter, or mm) to 1 m.
- **Infrared radiation:** Infrared light lies between 7 × 10-7 m (700 nanometers, or nm) and 1 mm, and is collected by optical equipment and put to visual use in "night vision" goggles.
- **Visible light:** The visible spectrum of light exists in a narrow band between about 4
× 10-7 m and 7 × 10-7 m (400 nm and 700 nm). From lowest to highest energy, and therefore longest to shortest wavelength, the spectral order of visible colors can be remembered by **"Roy G. Biv"**: red, orange, yellow, green, blue, indigo and violet.
- **Ultraviolet radiation:** Ultraviolet light (10-8 m to 4 × 10-7 m, or 10 nm to 400 nm) is used in tanning beds and is emitted by the sun; it's the kind of EM energy that causes sunburns and probably skin cancers.
- **X-rays:** This higher-energy radiation (10-11 to 10-8 m, or 0.01 to 10 nm) is used in diagnostics; large exposures can be unsafe to health.
- **Gamma rays:** The highest-energy EM is found zipping though outer space; this radiation has wavelengths shorter than 1/100th of a nanometer. The atmosphere fortunately filters out most of these.
Electromagnetic Spectrum Example Problem
Electromagnetic Spectrum Example Problem
You wake up on a planet where the speed of light is a sedate 60 meters per second, but physical laws in general are the same as on Earth. If the wavelengths of blue light and red light on this planet are 5 cm and 10 cm respectively, with yellow sitting exactly between them in the spectrum, what is the frequency of yellow light here?
The wavelength λ of yellow light on this planet must be half the distance between 5 and 10 cm, or 7.5 cm. Since λf = c = 300 m/s,
\(f=\frac{60}{0.075}=800\text{ cycles per second}\)
- Cycles per second has its own derived unit in physics, the hertz (Hz).
Cite This Article
MLA
Beck, Kevin. "Electromagnetic Spectrum: Frequencies, Wavelengths (W/ Diagrams & Examples)" sciencing.com, https://www.sciencing.com/electromagnetic-spectrum-frequencies-wavelengths-w-diagrams-examples-13721432/. 28 December 2020.
APA
Beck, Kevin. (2020, December 28). Electromagnetic Spectrum: Frequencies, Wavelengths (W/ Diagrams & Examples). sciencing.com. Retrieved from https://www.sciencing.com/electromagnetic-spectrum-frequencies-wavelengths-w-diagrams-examples-13721432/
Chicago
Beck, Kevin. Electromagnetic Spectrum: Frequencies, Wavelengths (W/ Diagrams & Examples) last modified August 30, 2022. https://www.sciencing.com/electromagnetic-spectrum-frequencies-wavelengths-w-diagrams-examples-13721432/