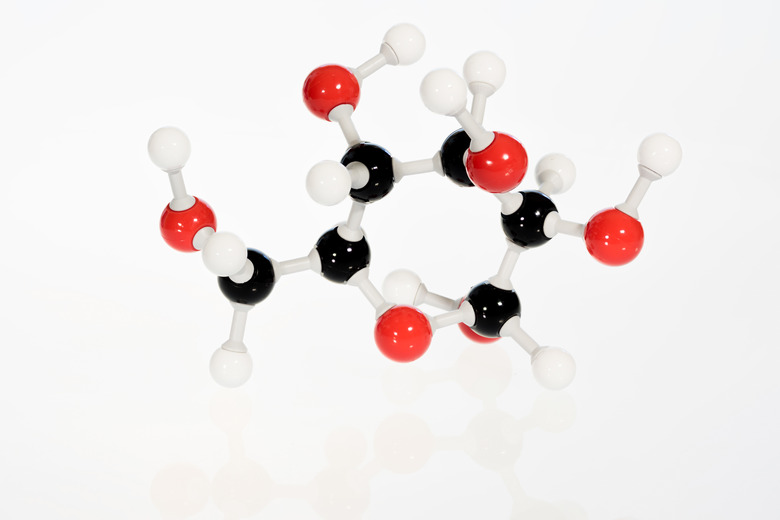
Equation For Glucose Metabolism
The cells in your body can break down or metabolize glucose to make the energy they need. Rather than merely releasing this energy as heat, however, cells store this energy in the form of adenosine triphosphate or ATP; ATP acts as a kind of energy currency that's available in a convenient form to meet the cell's needs.
Overall Chemical Equation
Overall Chemical Equation
Since the breakdown of glucose is a chemical reaction, it can be described using the following chemical equation: C6H12O6 + 6 O2 –> 6 CO2 + 6 H2O, where 2870 kilojoules of energy are released for each mole of glucose that's metabolized. Although this equation does describe the overall process, its simplicity is deceptive, because it conceals all the details of what's really taking place. Glucose isn't metabolized in a single step. Instead, the cell breaks glucose down in a series of small steps, each of which releases energy. The chemical equations for these appear below.
Glycolysis
Glycolysis
The first step in glucose metabolism is glycolysis, a ten-step process where a molecule of glucose is lysed or split into two three-carbon sugars which are then chemically altered to form two molecules of pyruvate. The net equation for glycolysis is as follows: C6H12O6 + 2 ADP + 2 [P]i + 2 NAD+ –> 2 pyruvate + 2 ATP + 2 NADH, where C6H12O6 is glucose, [P]i is a phosphate group, NAD+ and NADH are electron acceptors/carriers and ADP is adenosine diphosphate. Again, while this equation gives the overall picture, it also conceals a lot of the dirty details; since glycolysis is a ten-step process each step could be described using a separate chemical equation.
Citric Acid Cycle
Citric Acid Cycle
The next step in glucose metabolism is the citric acid cycle (also called the Krebs cycle or the tricarboxylic acid cycle). Each of the the two molecules of pyruvate formed by glycolysis are converted into a compound called acetyl CoA; through an 8-step process, these The net chemical equation for the citric acid cycle can be written as follows: acetyl CoA + 3 NAD+ + Q + GDP + [P]i + 2 H2O –> CoA-SH + 3 NADH + 3 H+ + QH2 + GTP + 2 CO2. A fuller description of all the steps involved is beyond the scope of this article; basically, however, the citric acid cycle donates electrons to two electron carrier molecules, NADH and FADH2, which can then donate these electrons to another process. It also produces a molecule called GTP that has similar functions to ATP in the cell.
Oxidative Phosphorylation
Oxidative Phosphorylation
In the last major step in glucose metabolism, the electron carrier molecules from the citric acid cycle (NADH and FADH2) donate their electrons to the electron transport chain, a chain of proteins embedded in the membrane of the mitochondria in your cells. Mitochondria are important structures that play a key role in glucose metabolism and in generating energy. The electron transport chain powers a process that drives the synthesis of ATP from ADP.
Effects
Effects
The overall results of glucose metabolism are impressive; for each molecule of glucose, your cell can make 38 molecules of ATP. Since it takes 30.5 kilojoules per mole to synthesize ATP, your cell successfully stores 40 percent of the energy released by breaking down glucose. The remaining 60 percent is lost as heat; this heat helps to maintain your body temperature. While 40 percent may sound like a low figure, it's considerably more efficient than many machines designed by humans. Even the best cars, for example, can only convert a quarter of the energy stored in gasoline into energy that moves the car.
References
- "Essential Cell Biology, 2nd Edition"; Alberts, Bray, Hopkin, Johnson, Lewis, Raff, Roberts, Walter; 2004.
- "Biology: A custom edition"; Campbell, Reece, Urry, Cain, Wasserman, Minorsky, Jackson; 2008.
Cite This Article
MLA
Brennan, John. "Equation For Glucose Metabolism" sciencing.com, https://www.sciencing.com/equation-glucose-metabolism-6646895/. 13 March 2018.
APA
Brennan, John. (2018, March 13). Equation For Glucose Metabolism. sciencing.com. Retrieved from https://www.sciencing.com/equation-glucose-metabolism-6646895/
Chicago
Brennan, John. Equation For Glucose Metabolism last modified August 30, 2022. https://www.sciencing.com/equation-glucose-metabolism-6646895/