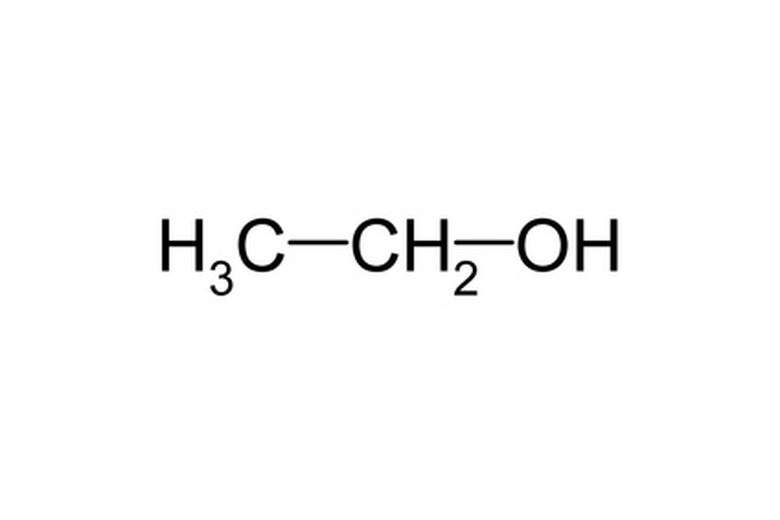
What Is Ethanolic Potassium Hydroxide?
Ethanolic potassium hydroxide is a solution of potassium hydroxide in ethanol. Potassium hydroxide is an inorganic, chemical compound made of one potassium atom bonded to an oxygen atom, which is itself bonded to a hydrogen atom. Ethanol is an alcohol.
Properties
Properties
Potassium hydroxide exists as a white solid powder, but is highly soluble in alcohols like ethanol. Ethanolic potassium hydroxide is highly acidic in nature. It is highly corrosive when handled. It is an effective conductor of heat, and highly flammable. It appears as a colorless liquid.
Manufacture
Manufacture
The manufacture of ethanolic potassium hydroxide involves creating potassium hydroxide and then dissolving the potassium hydroxide powder in ethanol. Potassium hydroxide is produced by boiling a solution of potash with slaked lime.
Uses
Uses
Ethanolic potassium hydroxide is used as a dessicant. It is also used in some electrical batteries, such as those used in television remote controls. It is used in the manufacture of soaps, the production of biodiesel and the production of other potassium compounds.
Cite This Article
MLA
, Thomas James. "What Is Ethanolic Potassium Hydroxide?" sciencing.com, https://www.sciencing.com/ethanolic-potassium-hydroxide-7483457/. 24 April 2017.
APA
, Thomas James. (2017, April 24). What Is Ethanolic Potassium Hydroxide?. sciencing.com. Retrieved from https://www.sciencing.com/ethanolic-potassium-hydroxide-7483457/
Chicago
, Thomas James. What Is Ethanolic Potassium Hydroxide? last modified March 24, 2022. https://www.sciencing.com/ethanolic-potassium-hydroxide-7483457/