How To Figure Out Protons, Neutrons, And Electrons
Atoms consist of a dense core, or nucleus, which contains positively charged particles called protons and uncharged particles called neutrons. Negatively charged electrons occupy somewhat confined regions of space outside the nucleus called orbitals. Protons and neutrons weigh almost 2,000 times more than electrons and therefore represent almost all of the mass of an atom. For any given element in the periodic table, the number of protons in the nuclei of its atoms is consistent. Every carbon atom, for example, contains six electrons. The number of electrons matches the number of protons in a neutral atom, but atoms can gain or lose electrons during chemical reactions. The number of neutrons also varies from one atom to the next. Chemists refer to atoms of the same element with differing numbers of neutrons as isotopes. Understanding these terms represents the key to determining the protons, neutrons and electrons in an isotope.
Step 1
Identify the mass number of the isotope from its symbol. By convention, scientists state the mass number of an isotope as a superscript number in front of the elemental symbol, such as 235U, or with a hyphen after the symbol, as in U-235.
Step 2
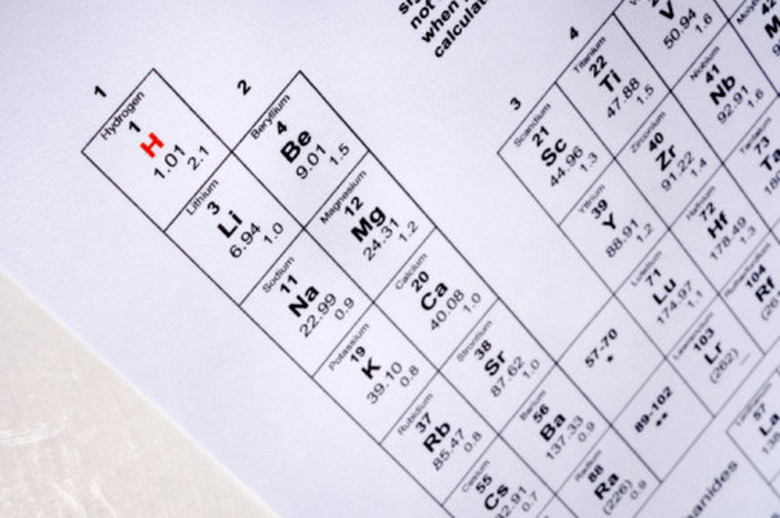
Determine the number of protons in the isotope's nucleus by locating its atomic number on the periodic table of the elements. The periodic table arranges elements by increasing atomic number. U, for example, represents the chemical symbol for uranium and it possesses an atomic number of 92. This means that all uranium atoms contain 92 protons in their nucleus.
Step 3
Calculate the number of electrons contained by the isotope by noting if its symbol includes a charge. The charge notation represents either a positive or negative number, usually written as a superscript after the chemical symbol, such as 235U(4+). This indicates that the uranium atom has lost four electrons. In the absence of a stated charge, the isotope possesses a charge of zero and its number of electrons equals its number of protons. Subtract positive charges from or add negative charges to the atomic number if the symbol contains a stated charge. The 235U(4+), for example, would contain 92 – 4 = 88 electrons.
Step 4
Find the number of neutrons in the isotope by subtracting the number of protons from the mass number given in the symbol. For example, 235U, which contains 92 protons, therefore contains 235 – 92 = 143 neutrons.
Things Needed
- Periodic table of the elements
- Calculator
Cite This Article
MLA
Brubaker, Jack. "How To Figure Out Protons, Neutrons, And Electrons" sciencing.com, https://www.sciencing.com/figure-out-protons-neutrons-electrons-8246096/. 24 April 2017.
APA
Brubaker, Jack. (2017, April 24). How To Figure Out Protons, Neutrons, And Electrons. sciencing.com. Retrieved from https://www.sciencing.com/figure-out-protons-neutrons-electrons-8246096/
Chicago
Brubaker, Jack. How To Figure Out Protons, Neutrons, And Electrons last modified March 24, 2022. https://www.sciencing.com/figure-out-protons-neutrons-electrons-8246096/