Five Types Of Atomic Models
Each successive model for atomic anatomy and construction was based on the previous one. Philosophers, theorists, physicists and scientists progressively developed the atomic paradigm over the course of many centuries. Several hypothetical models were proposed, modified and eventually rejected or accepted. Many scientists and thinkers made discoveries and conducted experiments to arrive at the currently accepted atomic model. The development of mathematics and specialized technology contributed greatly to the contemporary understanding of the nature of atoms.
Early Spherical Models
Early Spherical Models
Because atoms are too small to be seen, the first theoretical models were intellectual constructions based on the logical methods of inductive and deductive reasoning. The classical Greek philosopher Democritus was the first to propose the existence of atoms in 400 B.C. He reasoned that matter cannot be divided indefinitely and must consist of indivisible round particles called atoms. In 1800, John Dalton arrived at the same view of atomism by using the experimental method to study gases and compounds. His theory was called the solid sphere, or billiard ball, model.
Plum Pudding Model
Plum Pudding Model
In 1904 the British physicist J.J. Thompson posited the plum pudding, or raisin bun, model of atomism. It was based on knowledge of the recently discovered negatively charged subatomic particles called electrons. Thompson's experiments with cathode ray tubes prompted him to theorize the existence of tiny particles inside atoms that were fundamental parts of all atoms. His model envisioned the negative electrons, or plums, suspended inside a positively charged framework, or the pudding.
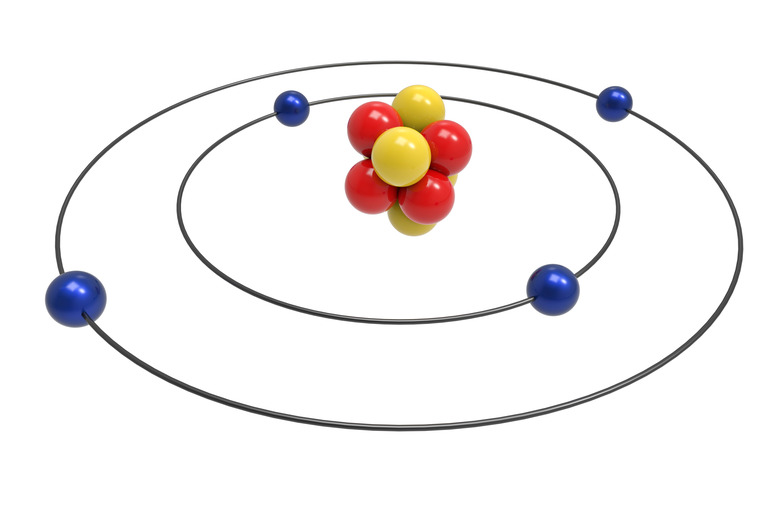
Two Planetary Orbit Models
Two Planetary Orbit Models
From 1910 to 1911, Ernest Rutherford proposed the planetary, or nuclear, model of the atom. He believed that atoms were composed mostly of empty space, with a dense nucleus. His experiments involved shooting alpha particles at gold foil. He concluded that the positive nucleus contains most of the atom's mass. With his orbit model, Niels Bohr refined the idea of the atom as a tiny solar system in 1913. Bohr's model had electrons orbiting the nucleus in shell-like layers.
Electron Cloud Model
Electron Cloud Model
Louis de Broglie and Erwin Schrodinger developed the electron cloud, or quantum mechanical, model. They based the model on the breakthroughs of the quantum mechanics branch of physics. Instead of electrons in fixed orbits, the cloud model has the orbits defined by a probability distribution around the nucleus. Depending on their observation and measurement, the electrons could be in many different places, sometimes simultaneously.
Cite This Article
MLA
Whitmer, Phil. "Five Types Of Atomic Models" sciencing.com, https://www.sciencing.com/five-types-atomic-models-7911352/. 13 March 2018.
APA
Whitmer, Phil. (2018, March 13). Five Types Of Atomic Models. sciencing.com. Retrieved from https://www.sciencing.com/five-types-atomic-models-7911352/
Chicago
Whitmer, Phil. Five Types Of Atomic Models last modified March 24, 2022. https://www.sciencing.com/five-types-atomic-models-7911352/