Heat Vs Temperature: What Are The Similarities & Differences? (W/ Graph)
People sometimes use the terms heat and temperature interchangeably. They associate heat with the word hot and understand temperature as also related to the "hotness" or "coldness" of something. Perhaps they'll say that the temperature on a spring day feels just right because it's just the right amount of heat.
In physics, however, these two quantities are quite distinct from each other. They are not measures of the same thing, and they do not have the same units, though they both can inform your understanding of thermal properties.
Internal Energy
Internal Energy
In order to understand heat and temperature on a fundamental level, it is first important to understand the concept of internal energy. While you may be familiar with objects having kinetic energy due to their motion, or potential energy due to their position, within a given object, the molecules themselves can also have a form of kinetic and potential energy.
This molecular kinetic and potential energy is separate from what you can see when looking at, say, a brick. A brick sitting on the ground appears to be motionless, and you might assume it to have no kinetic or potential energy associated with it. And indeed, it doesn't in the sense of your understanding of basic mechanics.
But the brick itself is composed of many molecules that individually are undergoing different types of small motions that you cannot see. The molecules may also experience potential energy due to their proximity to other molecules and the forces exerted between them. The total internal energy of this brick is the sum of the kinetic and potential energies of the molecules themselves.
As you've likely learned, energy is conserved. In the event that no friction or dissipative forces act on an object, mechanical energy is also conserved. That is, kinetic energy can change into potential energy and vice versa, but the total remains constant. When a force like friction acts, however, you may notice the total mechanical energy decreasing. This is because the energy took other forms such as sound energy or thermal energy.
When you rub your hands together on a cold day, you convert mechanical energy into thermal energy. That is, the kinetic energy of your hands moving against each other changed form and became kinetic energy of the molecules in your hands with respect to each other. The average of this kinetic energy in the molecules in your hands is what scientists define as temperature.
Definition of Temperature
Definition of Temperature
Temperature is a measure of average kinetic energy per molecule in a substance. Note that it is not the same as the internal energy of the substance because it does not include the potential energy and also is not a measure of the total energy in the substance. Instead, it is the total kinetic energy divided by the number of molecules. As such, it does not depend on how much of something you have (like total internal energy does) but rather on how much kinetic energy the average molecule in the substance is carrying around.
Temperature can be measured in many different units. Among these are Fahrenheit, which is most common in the U.S. and a few other places. On the Fahrenheit scale, water freezes at 32 degrees and boils at 212. Another common scale is the Celsius scale, used in many other places in the world. On this scale, water freezes at 0 degrees and boils at 100 degrees (which gives a pretty clear idea of how this scale was devised).
But the scientific standard is the Kelvin scale. While the size of an increment on the Kelvin scale is the same as a Celsius degree, the Kelvin scale starts at a temperature called absolute zero, which is where all molecular motion stops. In other words, it starts at the coldest possible temperature.
Zero degrees Celsius is 273.15 on the Kelvin scale. The Kelvin scale is the scientific standard for good reason. Suppose something is at 0 degrees Celsius. What would it mean to say that a second object is twice the temperature? Would that item also be 0 Celsius? Well on the Kelvin scale, this notion causes no problems, and it is precisely because it starts at absolute zero.
Definition of Heat
Definition of Heat
Consider two substances or objects at different temperatures. What does this mean? This means that, on average, the molecules in one of the substances (the higher-temperature one) are moving around with a greater average kinetic energy than the molecules in the lower-temperature substance.
If those two substances come in contact, not surprisingly, the energy begins to average out between the substances as microscopic collisions occur. The substance that was initially at the higher temperature will cool as the other substance rises in temperature until they are both the same temperature. Scientists call this final state thermal equilibrium.
The thermal energy that is transferred from the warmer object to the cooler object is what scientists call heat. Heat is the form of energy transferred between two materials that are at different temperatures. Heat always flows from the material with higher temperature to the material with lower temperature until thermal equilibrium is reached.
Since heat is a form of energy, the SI unit of heat is the joule.
Differences Between Heat and Temperature
Differences Between Heat and Temperature
As you've seen by the previous definitions, heat and temperature are indeed two distinct physical measures. These are just some of their differences:
**They are measured in different units.** The SI unit for temperature is the Kelvin, and the SI unit for heat is the joule.The Kelvin is considered a base unit, meaning it cannot be broken down into a combination of other fundamental units. The joule is equivalent to a kgm2/s2.
**They differ in their dependence on number of molecules.** Temperature is a measure of the average kinetic energy per molecule, which means it doesn't matter how much of a substance you have when you are talking temperature. The amount of heat energy that might be transferred between substances, however, very much depends on how much of each substance you have.
**They are different types of variables.** Temperature is known as a state variable. That is, it defines the state that a substance or object is in. Heat, on the other hand, is a process variable. It describes a process that is occurring – in this case, the energy being transferred. It doesn't make sense to talk about heat when everything is in equilibrium.
**They are measured differently.** Temperature is measured with a thermometer, which is typically a device that makes use of thermal expansion to change the reading on a scale. Heat, on the other hand, is measured with a calorimeter.
Similarities and Relationships Between Heat and Temperature.
Similarities and Relationships Between Heat and Temperature.
Heat and temperature are not entirely unrelated to each other, however:
**They are both important quantities in thermodynamics.** The study of thermal energy relies on the ability to measure temperature as well as the ability to keep track of heat transfers.
**Heat transfer is driven by temperature differences.** When two objects are at different temperatures, heat energy will transfer from the warmer one to the cooler one until thermal equilibrium is reached. As such, these temperature differences are the driver of heat transfer.
**They tend to increase and decrease together.** If heat is added to a system, temperature goes up. If heat is removed from a system, temperature goes down. (One exception to this occurs with phase transitions, in which case heat energy is used to cause a phase transition instead of a change in temperature.)
**They are related to each other by an equation.** Heat energy Q is related to a change in temperature ΔT via the equation Q = mcΔT where m is the mass of the substance and c is its specific heat capacity (that is, a measure of the amount of heat energy required to raise a unit mass by a degree Kelvin for a particular substance.)
Heat, Temperature and Total Internal Energy
Heat, Temperature and Total Internal Energy
Internal energy is the total internal kinetic and potential energy, or thermal energy in a material. For an ideal gas, in which potential energy between molecules is negligible, internal energy E is given by the formula E = 3/2nRT where n is the number of moles of the gas and the universal gas constant R = 8.3145 J/molK.
The relationship between internal energy and temperature shows that, not surprisingly, as temperature increases, thermal energy increases. The internal energy also becomes 0 at absolute 0 Kelvin.
Heat comes into the picture when you start looking at changes in internal energy. The first law of thermodynamics gives the following relationship:
\(\Delta E = Q – W\)
where Q is the heat added to the system and W is the work done by the system. In essence, this is a statement of conservation of energy. When you add heat energy, the internal energy increases. If the system does work on its surroundings, the internal energy decreases.
Temperature as a Function of Heat Energy
Temperature as a Function of Heat Energy
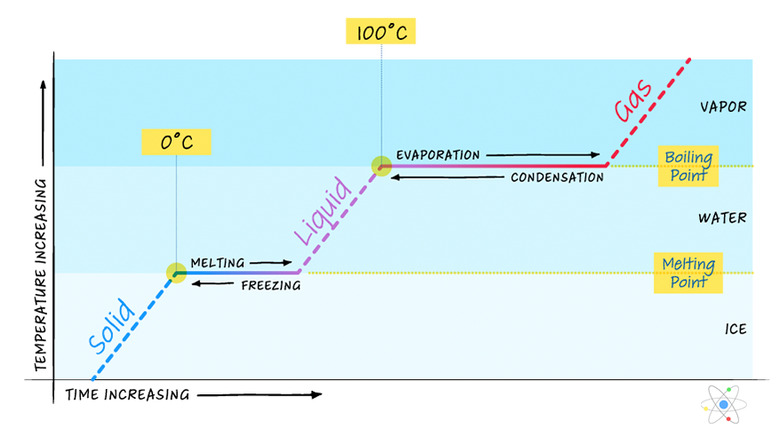
As mentioned previously, heat energy added to a system typically results in a corresponding temperature increase unless the system is undergoing a phase change. To look at this more closely, consider a block of ice that starts out below freezing as heat energy is added at a constant rate.
If heat energy is added continuously while the block of ice warms up to freezing, undergoes a phase change to become water and then continues to warm up until it reaches boiling, where it undergoes another phase change to become steam, the graph of temperature vs. heat will look like the following:
While the ice is below freezing, there is a linear relationship between heat energy and temperature. This is not surprising as it should be, given the equation Q = mcΔT. Once the ice reaches the freezing temperature, however, any heat energy added must be used to help it change phase. The temperature remains constant even though heat is still being added. The equation that relates heat energy to mass during a phase change from solid to liquid is the following:
\(Q=mL_f\)
where _Lf_ is the latent heat of fusion – a constant relating how much energy is required per unit mass to cause the change from solid to liquid.
So, until a quantity of heat equal to _mLf_ has been added, the temperature remains constant.
Once all the ice has melted, the temperature again rises linearly until it reaches the boiling point. Here again a phase change occurs, this time from liquid to gas. The equation relating heat to mass during this phase change is very similar:
\(Q=mL_v\)
where _Lv_ is the latent heat of vaporization – a constant relating how much energy is required per unit mass to cause the change from liquid to gas. So the temperature once again remains constant until enough heat energy has been added. Note that it remains constant for longer this time. That is because _Lv_ is typically higher than _Lf_ for a substance.
The last part of the graph again shows the same linear relationship as before.
Cite This Article
MLA
TOWELL, GAYLE. "Heat Vs Temperature: What Are The Similarities & Differences? (W/ Graph)" sciencing.com, https://www.sciencing.com/heat-vs-temperature-what-are-the-similarities-differences-w-graph-13722757/. 28 December 2020.
APA
TOWELL, GAYLE. (2020, December 28). Heat Vs Temperature: What Are The Similarities & Differences? (W/ Graph). sciencing.com. Retrieved from https://www.sciencing.com/heat-vs-temperature-what-are-the-similarities-differences-w-graph-13722757/
Chicago
TOWELL, GAYLE. Heat Vs Temperature: What Are The Similarities & Differences? (W/ Graph) last modified March 24, 2022. https://www.sciencing.com/heat-vs-temperature-what-are-the-similarities-differences-w-graph-13722757/