How Can Parts Of A Solution Be Separated By Chromatography?
A solution is a homogeneous mixture of at least two substances. When chemists need to determine what components are present in a solution or other mixture, they often use a technique called chromatography. Chromatography is a process that pulls apart the components of a mixture so that they can be identified. This a common technique used in research, as well as in other industries such as medicine and forensics. There are several types of chromatography, but they all work because of the same chemistry principles.
TL;DR (Too Long; Didn't Read)
Chromatography is a scientific process that pulls apart the components of a solution or other mixture so that they can be identified. Many different materials are used to accomplish this, but every type of chromatography includes a "stationary phase" material that doesn't move, and a "mobile phase" material that travels past the stationary phase, carrying the solution with it. Based on their molecular properties, some chemicals in the solution will travel farther with the stationary phase than others. Once they are spread out, the chemicals can be identified by how far they traveled and their individual properties.
Paper Chromatography
Paper Chromatography
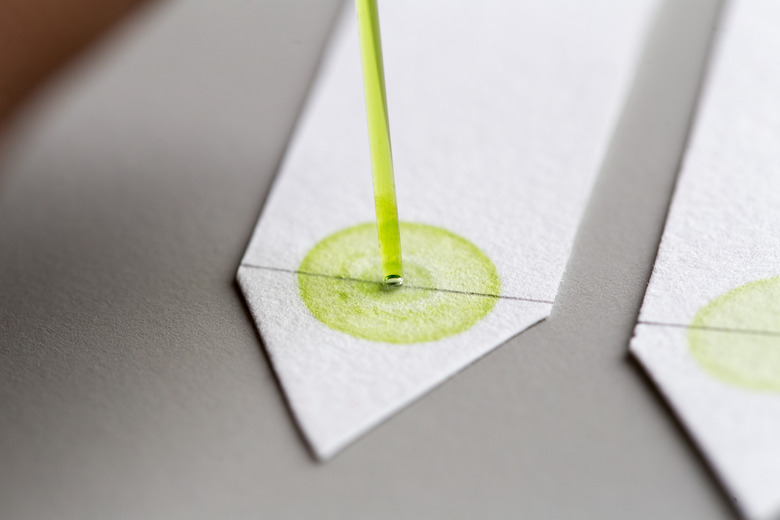
A simple way to understand how chromatography separates the parts of a solution is to think about what happens when a piece of paper with writing on it gets wet. The ink spreads out across the paper in streaks. Everyone has experience with this unintentional version of paper chromatography. The solution is the ink, and the chemicals in the ink separate when the paper gets wet. The same method is used to separate chemicals in solutions other than ink.
In this method, a pencil line is drawn horizontally across the paper at the very bottom, and a dot of the solution being tested is added. When it dries, the paper is hung vertically over a dish. Enough of a liquid solvent is added to the dish to reach the bottom of the paper, but not the pencil line. The solvent begins to climb the paper, and when it reaches the dot of solution, it begins to carry the chemicals in the solution with it. In paper chromatography, the paper is the element of the experiment that stays still, so it is called the "stationary phase." The solvent moves up the paper, bringing the solution being tested with it, so the solvent is known as the "mobile phase."
Adsorption
Adsorption
Molecules in both the solvent and the solution interact with the molecules in the paper. They get temporarily stuck on the surface of the paper, in a process called adsorption. Unlike absorption, adsorption is not permanent. Eventually, the molecules break free and continue climbing the paper, but the molecules in each chemical component bond differently with the molecules in the paper. Some become unstuck more quickly, and travel up the paper more quickly than the other chemicals' molecules. When the solvent has almost reached the top of the paper, a pencil line is drawn to mark its location before it evaporates. The positions of the chemical dots that separated from the original solution are also marked.
If the chemicals are colorless, other techniques can reveal them, such as shining ultraviolet light on the paper to show the dots, or spraying a chemical that will react with the dots and give them color. Sometimes the distance each dot traveled is measured relative to the distance the solvent traveled. This ratio is known as the retention factor, or the Rf value. It is useful for identifying a mixture's components because the Rf value can be compared to those of known chemicals.
Principles of Chromatography
Principles of Chromatography
Paper chromatography is only one kind of chromatography. In other forms of chromatography, the stationary phase could be a number of other materials, such as a plate of glass or aluminum coated with a liquid, a jar filled with liquid or a column filled with solid particles like silica crystals. The mobile phase might not even be a liquid solvent, but a gaseous "eluant." All chromatography works by doing the same thing with many different materials and techniques – a mobile phase is moved across or through a stationary phase. The solution is separated into its components based on how much each part of the solution dissolves into the mobile phase and is carried along, and how much it sticks to the adsorbent stationary phase and slows down.
Cite This Article
MLA
E., Rebecca. "How Can Parts Of A Solution Be Separated By Chromatography?" sciencing.com, https://www.sciencing.com/how-can-parts-of-a-solution-be-separated-by-chromatography-13710470/. 27 March 2018.
APA
E., Rebecca. (2018, March 27). How Can Parts Of A Solution Be Separated By Chromatography?. sciencing.com. Retrieved from https://www.sciencing.com/how-can-parts-of-a-solution-be-separated-by-chromatography-13710470/
Chicago
E., Rebecca. How Can Parts Of A Solution Be Separated By Chromatography? last modified March 24, 2022. https://www.sciencing.com/how-can-parts-of-a-solution-be-separated-by-chromatography-13710470/