How Do Batteries Work? Parts, Types & Terminology (W/ Diagram)
Without batteries, there would be no cell phones, watches, tablets, hearing aids, flashlights, electric cars or communication satellites – and the list goes on. The first battery was invented over 200 years ago, and ever since then, these ingenious devices have become indispensable in our daily lives.
What Is a Battery?
What Is a Battery?
Simply speaking, a battery is any device that can provide a portable temporary source of electrical energy.
In an electric circuit, batteries serve as a power source by creating a potential difference that drives the flow of electric current. As current passes through the circuit, it transfers energy to any devices connected to it. In such a circuit, the type of current that flows is direct current. In other words, the current that flows goes in one, continuous direction.
Conversely, power supplied by a power plant is accessed via the outlets in your home and is in the form of alternating current. This type of current alternates direction with a certain frequency in order to power devices.
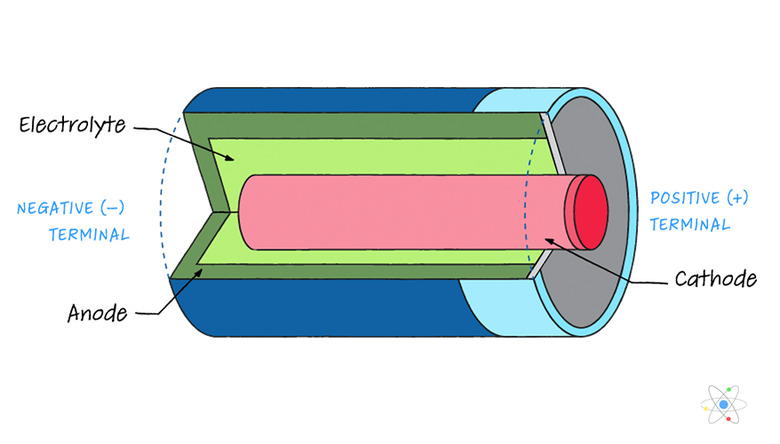
How Batteries Work
How Batteries Work
A typical battery is composed of one or more cells that have a cathode (positive terminal) on one end and an anode (negative terminal) on the other end. Chemical reactions contained within cause a buildup of electrical charge at the terminals, producing an electric potential across the nodes via the release of chemical energy.
The chemical reactions in the battery cause electron buildup at the anode. This creates an electric potential between the cathode and anode. The electrons want to make it to the cathode in order to neutralize the charge, but they cannot do so by traveling through the electrolytic material inside the battery itself. Instead, electrons flow easily through a conducting wire connecting the anode to the cathode.
Eventually, the chemical processes creating the surplus of electrons in the anode come to a stop, and the battery dies. With rechargeable batteries (also called secondary batteries), however, this process can be reversed by connecting the batteries to battery chargers after they die. Recharging a battery reverses the flow of electrons by using another power source. The chemical processes in the battery are able to reverse due to this added energy, and the battery will once again be able to power a circuit on its own.
Create Your Own Lemon Battery!
Create Your Own Lemon Battery!
An excellent way to better understand how a battery works is to create your own battery at home with a lemon, a zinc nail and a copper coin, and use it to power a small light bulb.
Insert a copper coin into one side of the lemon, and insert the galvanized (zinc-coated) nail into the other side (making sure the two items do not touch inside the lemon). The nail will serve as the positive electrode (cathode), and the coin will be the negative electrode (anode). The lemon's juice serves as the electrolyte. You can then connect a voltmeter across your lemon battery to see how much voltage it creates. If necessary, you can connect several lemon batteries in series to create enough voltage to power a small light bulb.
Different Types of Batteries
Different Types of Batteries
Batteries come in all different shapes, sizes, compositions and voltages. Some of the most common types are:
- Rechargeable batteries used in common household electronic devices. These include lithium-ion batteries, nickel cadmium and nickel metal hydride (NiMH). The names of the batteries indicate the electrolytes they contain.
- Lead-acid batteries are also rechargeable, but they are used for more heavy-duty applications (as car batteries, for example).
- Batteries that are not typically rechargeable include alkaline batteries or dry cell zinc-carbon batteries.
Cite This Article
MLA
TOWELL, GAYLE. "How Do Batteries Work? Parts, Types & Terminology (W/ Diagram)" sciencing.com, https://www.sciencing.com/how-do-batteries-work-parts-types-terminology-w-diagram-13721182/. 2 March 2020.
APA
TOWELL, GAYLE. (2020, March 2). How Do Batteries Work? Parts, Types & Terminology (W/ Diagram). sciencing.com. Retrieved from https://www.sciencing.com/how-do-batteries-work-parts-types-terminology-w-diagram-13721182/
Chicago
TOWELL, GAYLE. How Do Batteries Work? Parts, Types & Terminology (W/ Diagram) last modified August 30, 2022. https://www.sciencing.com/how-do-batteries-work-parts-types-terminology-w-diagram-13721182/