How To Find Isotopes
Every atom has a certain number of protons, electrons and neutrons. Protons carry a positive charge, electrons carry a negative charge and neutrons do not carry a charge. Protons and neutrons make up the nucleus or central part of the atom. Electrons orbit around the nucleus. Most atoms have isotopes that occur naturally. An isotope is an atom with a different number of neutrons, but the same number of protons and electrons. Each element has a standard number of neutrons that can be found by looking at a periodic table. From the periodic table, you will get the atomic number on the top left corner of the box. This is the number of protons. The atomic weight of the element can be found on the bottom of the box on the periodic table.
How to Find the Most Common Isotope
Step 1
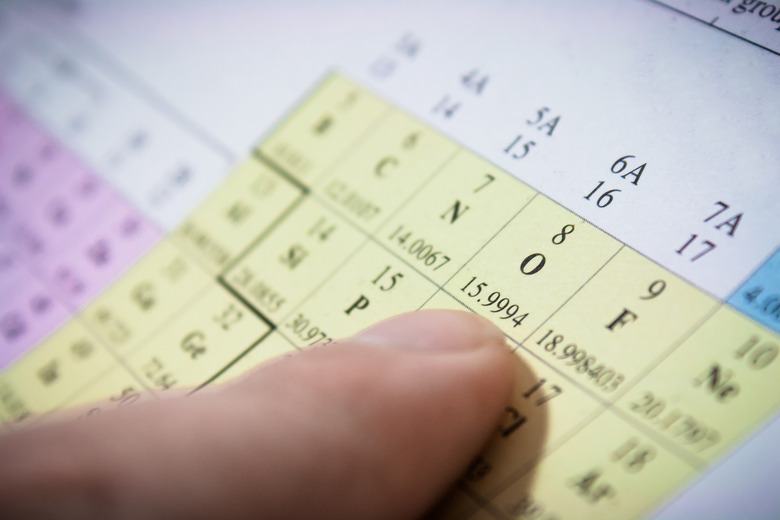
Find the element on the periodic table. Record the atomic weight (on the bottom) and the atomic number (top left).
Step 2
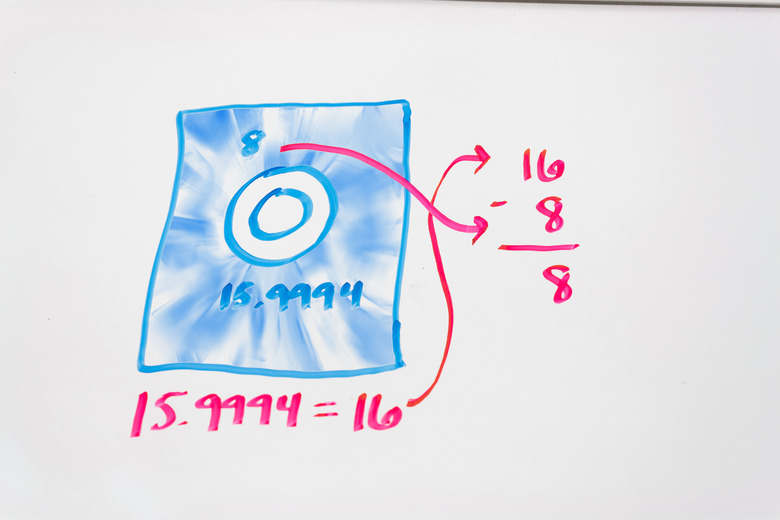
Round the atomic weight to the nearest whole number. If the decimal is .5 or higher, round up, if it is .49 or lower, round down.
Step 3
Subtract the atomic number (the number of protons) from the rounded atomic weight. This gives you the number of neutrons in the most common isotope.
Step 4
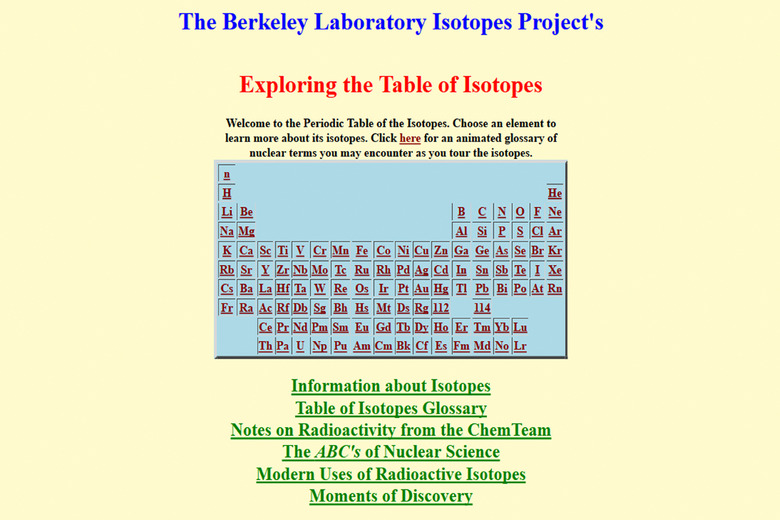
Use the interactive periodic table at The Berkeley Laboratory Isotopes Project to find what other isotopes of that element exist.
TL;DR (Too Long; Didn't Read)
It is helpful to write out each step and clearly label each value so that if you find you have made an error, it will be easier to check your work.
Warning
Finding the most common isotope is a fairly simple calculation. It is also possible to reverse the process and use the isotope values to find the atomic weight.
Cite This Article
MLA
Malone, Maureen. "How To Find Isotopes" sciencing.com, https://www.sciencing.com/how-to-find-isotopes-12293198/. 25 October 2017.
APA
Malone, Maureen. (2017, October 25). How To Find Isotopes. sciencing.com. Retrieved from https://www.sciencing.com/how-to-find-isotopes-12293198/
Chicago
Malone, Maureen. How To Find Isotopes last modified March 24, 2022. https://www.sciencing.com/how-to-find-isotopes-12293198/