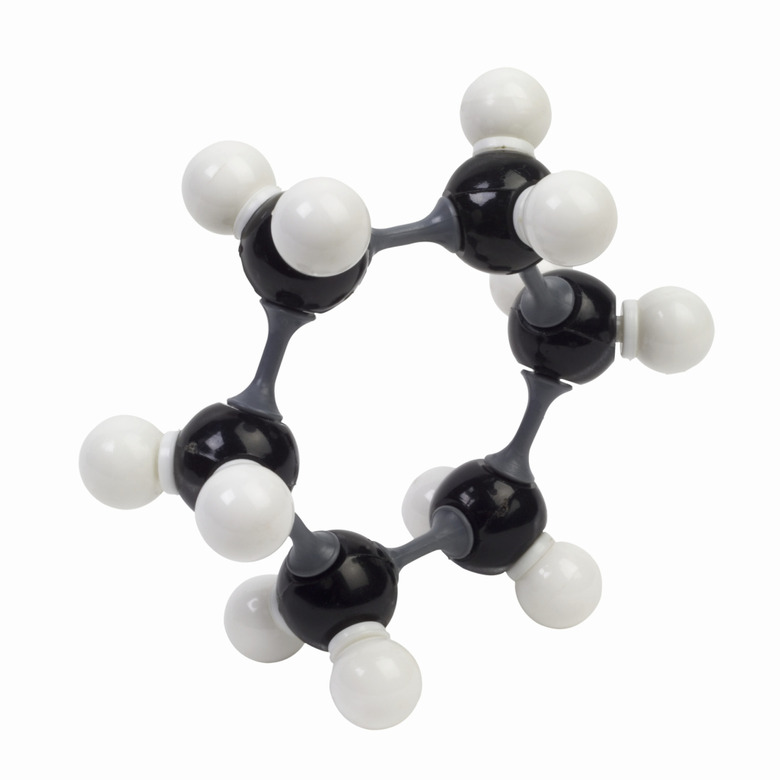
What Is A Hydrocarbon Chain?
A hydrocarbon chain is a molecule that consists of entirely hydrogen and carbon. They are the simplest of the organic compounds and may be a liquid, gas or solid. There are many types of hydrocarbon chains, including alkanes, alkenes, alkynes, cycloalkanes and arenes. They can be branched, linear, or cyclical. Hydrocarbon chains are ubiquitous in nature. They are non-polar, which means they do not mix with water.
Carbon's Valence Shell
Carbon's Valence Shell
The simplest hydrocarbon is methane, which is a single central carbon atom bound to four hydrogen atoms. The central carbon atom can form no greater than four other bonds because it has only four valence electrons. Valence electrons are free electrons on the outer shell of the atom that are available to bind, or make pairs, with valence electrons on other atoms to form molecules. While saturated carbon chains have all four valence electrons occupied around each carbon, some hydrocarbons may have unsaturated points where only two or three bonds form around the central carbon. Those unsaturations can be in the form of double or triple bonds to other carbons in places where hydrogen is absent so that all four valence electrons are still occupied.
Naming of Hydrocarbons
Naming of Hydrocarbons
Hydrocarbons are named using a prefix based on the number of carbons in the chain and a suffix indicating the types of bonds contained within them. Single, double, and triple bonds are called alkanes, alkenes, and alkynes respectively. For the compound "ethane," which is a gas, the prefix "eth-" indicates two carbons in the chain, and the suffix "-ane" indicates that it contains only single bonded carbons and hydrogens. A nine-carbon compound containing double bonds is called nonene. Hexane is an example of a six-carbon molecule with only single bonds. If the molecule is a ring, it begins with the prefix "cyclo-" such as with cyclohexane, a six-carbon ring with all single bonds.
Other Naming Rules
Other Naming Rules
When a hydrocarbon is attached to another molecule as a "functional group," the prefix also contains a "-yl" ending. For example, when ethane is attached to another molecule, it is called an ethyl group. When a compound has more than one unsaturation, such as a double bond, the number of the carbon where the double bond originates is included in the name using a number. For example, a butene molecule with a double bond between the first and second carbons is called 1-butene. Lastly, special hydrocarbons called arenes, or aromatic hydrocarbons, are rings that have alternating single and double bonds.
Examples of Hydrocarbons
Examples of Hydrocarbons
Hydrocarbons have many modern applications. Natural rubber is a type of hydrocarbon that consists of alternating double and single bonded carbons. Essential oils such as menthol and camphor are in a class of ring-shaped hydrocarbons called terpenoids and consist of 10 carbons and at least one double bonded carbon pair. While menthol can be found in cigarettes, and camphor is used as moth repellant, some types of fragrant essential oils are used in medicine and perfume. Gasoline, while it is not a pure hydrocarbon, contains a mixture of hydrocarbons of varying lengths including heptane, isooctane, cyclooctane and ethyl benzene. Numerous solvents, such as ethanol and benzene, are often used in the production of pharmaceuticals.
References
- Organic Chemistry: John E. McMurry
- Purdue University College of Science: Hydrocarbons
- Elmhurst College: Gasoline
- The Center for Disease Control and Prevention: Organic Solvents
Cite This Article
MLA
Riggio, Gina. "What Is A Hydrocarbon Chain?" sciencing.com, https://www.sciencing.com/hydrocarbon-chain-15056/. 24 April 2017.
APA
Riggio, Gina. (2017, April 24). What Is A Hydrocarbon Chain?. sciencing.com. Retrieved from https://www.sciencing.com/hydrocarbon-chain-15056/
Chicago
Riggio, Gina. What Is A Hydrocarbon Chain? last modified March 24, 2022. https://www.sciencing.com/hydrocarbon-chain-15056/