Which Liquids Boil At A Lower Gas Temperature Than Water?
Boiling points of substances vary depending on their structure at the molecular level. We're all familiar with boiling point of water at standard pressure — 100 degrees Celsius or 212 degrees Fahrenheit. Many of the substances you think of as gases, however, are only gases because their boiling points are well below room temperature. Even some substances that are liquids at room temperature, like ethanol, have lower boiling points than water.
Atmosphere
Atmosphere
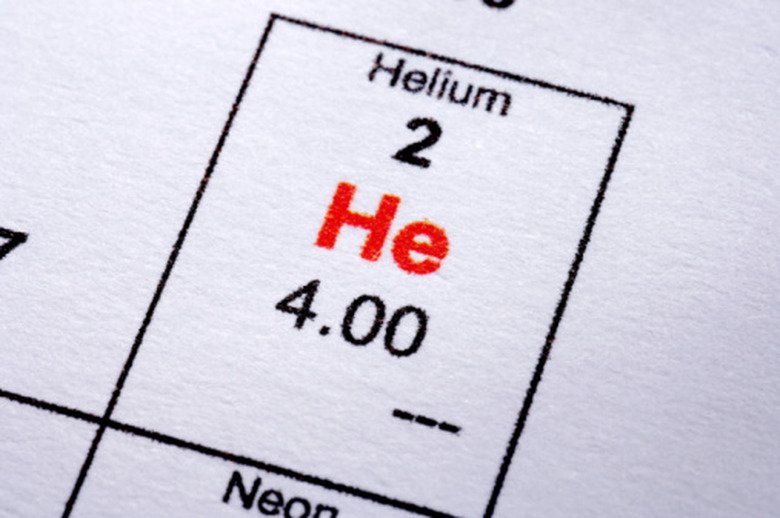
Nitrogen (N2), carbon dioxide, oxygen (O2), helium, chlorine (Cl2) and hydrogen are all familiar examples of substances that boil at much lower temperatures than water. Liquid helium has the lowest boiling point of all — about -452 degrees Fahrenheit, only 4.2 degrees Celsius above absolute zero. Although these substances are called gases, it's important to remember that no substance can be defined as a "gas" or a "liquid" except at a specific temperature. Liquid, solid and gas are all just different states of matter, and a substance can inhabit any one of these three states, depending on the temperature and pressure.
Nonpolar Hydrocarbons
Nonpolar Hydrocarbons
Water has a dipole moment, meaning it's polar because there's weak partial negative charge on oxygen and weak partial positive charge on hydrogens. Hydrocarbon compounds like those found in gasoline, however, are nonpolar. Interactions called London dispersion forces hold nonpolar molecules together in the solid or liquid phase; these London forces become stronger as the size of the molecules increases. Consequently, many smaller nonpolar molecules like the components of gasoline boil at a lower temperature than water because the intermolecular interactions are weaker.
Alcohols
Alcohols
Like water molecules, alcohols are polar and can also form a special kind of bond called a hydrogen bond. Water molecules, however, can form two hydrogen bonds, whereas an alcohol can only form one. Alcohols tend to have a higher boiling point than hydrocarbons of the same size but a lower boiling point than water. That's how you make liquor like whiskey: through distillation to increase the ethanol content.
Other Molecules
Other Molecules
Many other molecules have lower boiling points than water. One notable example is a class of molecules called ethers, which have an oxygen bonded to two carbons; they are slightly polar but not as polar as water or alcohols and cannot form hydrogen bonds, so they typically have lower boiling points. Another example is ammonia, which is typically sold dissolved in water. It boils below 0 degrees Celsius and at room temperature is found as a gas, albeit one that dissolves readily.
References
- "Organic Chemistry, Structure and Function, 6th Edition"; Peter Vollhardt and Neil Schore; 2011
- Georgia State University Hyperphysics: Liquid Helium
Cite This Article
MLA
Brennan, John. "Which Liquids Boil At A Lower Gas Temperature Than Water?" sciencing.com, https://www.sciencing.com/liquids-lower-gas-temperature-water-8194412/. 24 April 2017.
APA
Brennan, John. (2017, April 24). Which Liquids Boil At A Lower Gas Temperature Than Water?. sciencing.com. Retrieved from https://www.sciencing.com/liquids-lower-gas-temperature-water-8194412/
Chicago
Brennan, John. Which Liquids Boil At A Lower Gas Temperature Than Water? last modified March 24, 2022. https://www.sciencing.com/liquids-lower-gas-temperature-water-8194412/