How To Make A 3D Model Of An Atom
An atom, as you have perhaps heard, is often called the smallest ordinary component of matter that exists. Atoms consist of individual components (some of which include their own subatomic components), but an atom, which is about 1 × 10-10 m wide, is the smallest particle that retains the individual properties of a larger whole.
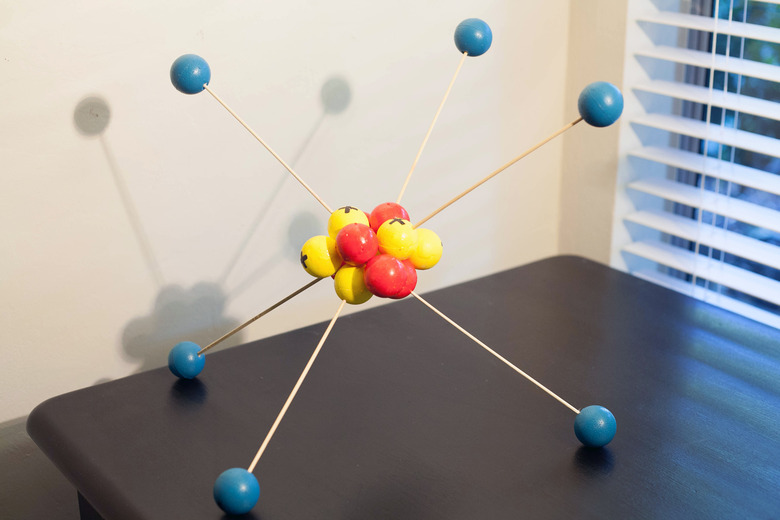
The approximate basic shapes of atoms are known, with the protons and neutrons clumped together in a ball in the nucleus and the far smaller electrons zipping about some relatively vast distance from the tiny (about 1 × 10−15 m wide) nucleus. This allows you to construct three-dimensional, or "3D," models of atoms in the class or at home.
Atom Basics
Atom Basics
Individual atom types are called elements, and each element has a name, e.g., hydrogen, rubidium or neon. These named elements are numbered one through 118 on the periodic table of the elements. These atomic numbers correspond to the number of protons and electrons, as well as the approximate average number of neutrons, in each element.
You can get an estimate of neutron number for an element by subtracting its atomic number from its atomic mass, listed at the center bottom of element boxes in most periodic tables. While protons and neutrons are on the order of only 10−27 kg, an electron is some 2,000 times less massive.
The positively charged protons and neutral neutrons are a considerable distance from the much smaller, negatively charged electrons, rather like planets in the solar system, except that the position of any electron cannot be precisely known at any instant, only assigned a certain probability. For 3D atom model purposes, though, think of an atom as similar to a tiny system of a central astronomical body and its satellites.
Things You'll Need
Things You'll Need
Luckily, you won't need any fancy equipment, but it should be visually striking. Any type of rubber or similar balls will work; it is helpful if these come in different colors, and it is helpful if you can write on them with magic marker (and even better if you can easily erase the ink later).
From the periodic table, O has an atomic number of 8 and an atomic mass of very close to 16.0. A 3D oxygen atom model or picture would therefore show eight protons and eight neutrons in the nucleus and eight electrons somewhere outside the nucleus. The protons and neutrons could be balls distinguished only by their color; the electrons are best represented in this scheme by far smaller balls.
Atom Model Project Ideas
Atom Model Project Ideas
Argon atom 3D model: From the table of elements, argon (Ar) has an atomic number of 18 and an atomic mass of 39.95. This means that a typical argon atom can be assumed to have about 40 − 18 = 22 neutrons to go with its 18 protons.
- The different manifestations of the same atom that vary only in neutron number are called isotopes. The proton-plus-neutron number of a given isotope is given using a left superscript next to the element symbol.
This atom could therefore be represented by 18 proton balls (pink) and 22 neutron balls (navy) in a globular cluster, with a ring of 18 evenly spaced electron balls (or marbles) surrounding this core. In reality, the electrons would surround the nucleus in a "cloud," not a planar ring like the solar system, and would assume rough geometric shapes in accordance with their energy levels, or shells.
Calcium atom model: Calcium has two more protons and therefore two more electrons than argon, so you can easily build it on a "base" of an argon atom. However, it is helpful to know something about valence electrons to understand why the two new electrons both belong farther from the nucleus than the electrons in argon.
This is because argon is a noble gas, meaning that its outermost electron shell is filled. Its rightmost position on the periodic table indicates this. Calcium, in column 2 of the table has two valence electrons. This topic, however, goes beyond the scope of atom project ideas for school.
Cite This Article
MLA
Beck, Kevin. "How To Make A 3D Model Of An Atom" sciencing.com, https://www.sciencing.com/make-3d-model-atom-5887341/. 9 March 2020.
APA
Beck, Kevin. (2020, March 9). How To Make A 3D Model Of An Atom. sciencing.com. Retrieved from https://www.sciencing.com/make-3d-model-atom-5887341/
Chicago
Beck, Kevin. How To Make A 3D Model Of An Atom last modified March 24, 2022. https://www.sciencing.com/make-3d-model-atom-5887341/