How To Make A 3D Model Of Sodium
The element sodium is in the alkali metal group of the periodic table. It comprises approximately 2.8 percent of Earth's crust. In appearance, sodium is a soft silvery-white metal. Its atomic formula is Na. Creating a 3D model of the sodium atom provides an interactive hands-on experience that is both insightful and informative.
Background Information
Background Information
Three-dimensional models are visualized replicas of what an element's atomic structure might look like. They are based on the Bohr model of the atom. Danish physicist Niels Bohr (1885-1962) was the first to conceptualize the planetary model illustration of the atom. The Bohr model essentially divides the atom into an electron cloud and a nucleus. The nucleus contains the protons and neutrons. The electron cloud is where the electrons can be found. Electrons revolve around the atomic nucleus in stable orbits, or shells. While the Bohr model has undergone numerous modifications over the years, its underlying principles are still relied upon when teaching the fundamentals of atomic structure. For this reason, the Bohr model is used to illustrate how to devise a 3D model of the sodium atom.
Things Needed
- Arts and crafts cotton balls of three different colors to correspond with protons, neutrons and electrons
- Cardboard or thick posterboard
- Scissors
- Glue
- String
TL;DR (Too Long; Didn't Read)
Although there will be three different colors of cotton balls, note that protons and neutrons are roughly the same size, whereas electrons are smaller. Thus, make certain to have two different-sized cotton balls, with the larger ones representing the protons and neutrons and the smaller ones representing the electrons.
1. Gather the Materials for the 3D Sodium Model
Assemble the materials needed. These include arts and crafts cotton balls of different hues to represent the electrons, protons and neutrons. Protons and neutrons are equal in size, while electrons are smaller than both protons and neutrons. Hence, choose appropriately sized craft cotton balls to simulate those size differences. As for the "shells" of the electron cloud, they can be cut, using scissors, from cardboard or thick posterboard. Similarly, make certain to have string on hand. Use string to tie the electron shells in concentric circles to simulate orbits around the nucleus. Glue attaches the craft cotton balls to their corresponding regions.
2. Construct the Nucleus
Locate sodium on the periodic table to determine its atomic number. An element's atomic number will hint at the number of protons and the number of electrons it has. Remember that a stable, neutral atom has equal numbers of electrons to protons. Consequently, sodium's atomic number of 11 indicates that it has an equal number of 11 protons and 11 electrons.
3. Determine the Number of Neutrons
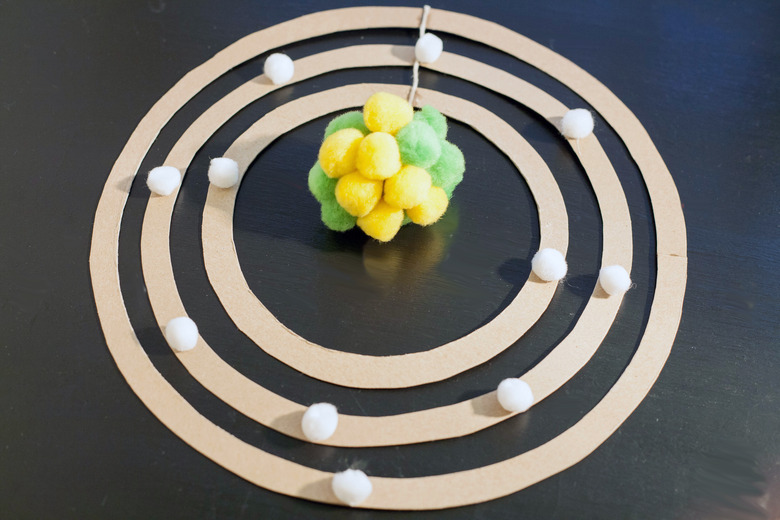
Find the number of neutrons that sodium has, by first looking at its atomic weight on the periodic table. Sodium has the atomic weight of about 23. That means its nucleus has 12 neutrons, since 23 minus 11 protons equals 12 neutrons. Now that you have determined the number of protons and neutrons, then choose to create a nucleus of 11 yellow-colored protons and 12 green-colored neutrons, as depicted in the photo.
4. Build the Electron Shells
Craft the electron shells that surround the sodium atom's nucleus. In chemistry and atomic physics, electron shells correspond to the principal energy levels where electrons orbit around the atomic nucleus. Moreover, each of these shells is occupied by a fixed number of electrons. The general rule of thumb is that the nth shell can hold up to 2(n-squared) electrons. Thus, the first shell, which is the innermost shell, holds a maximum of two electrons. Next, the second shell holds a maximum of eight electrons. That is followed by the third shell, which holds a maximum of 18 electrons. Because sodium has 11 electrons, its first shell will be fully occupied by two electrons. That is followed by its second shell being fully occupied by eight electrons, thus leaving its third shell with just one electron, as seen in the illustration provided.
Cite This Article
MLA
Agravante, Mariecor. "How To Make A 3D Model Of Sodium" sciencing.com, https://www.sciencing.com/make-3d-model-sodium-5646441/. 11 April 2018.
APA
Agravante, Mariecor. (2018, April 11). How To Make A 3D Model Of Sodium. sciencing.com. Retrieved from https://www.sciencing.com/make-3d-model-sodium-5646441/
Chicago
Agravante, Mariecor. How To Make A 3D Model Of Sodium last modified March 24, 2022. https://www.sciencing.com/make-3d-model-sodium-5646441/