How Benzene Is Made
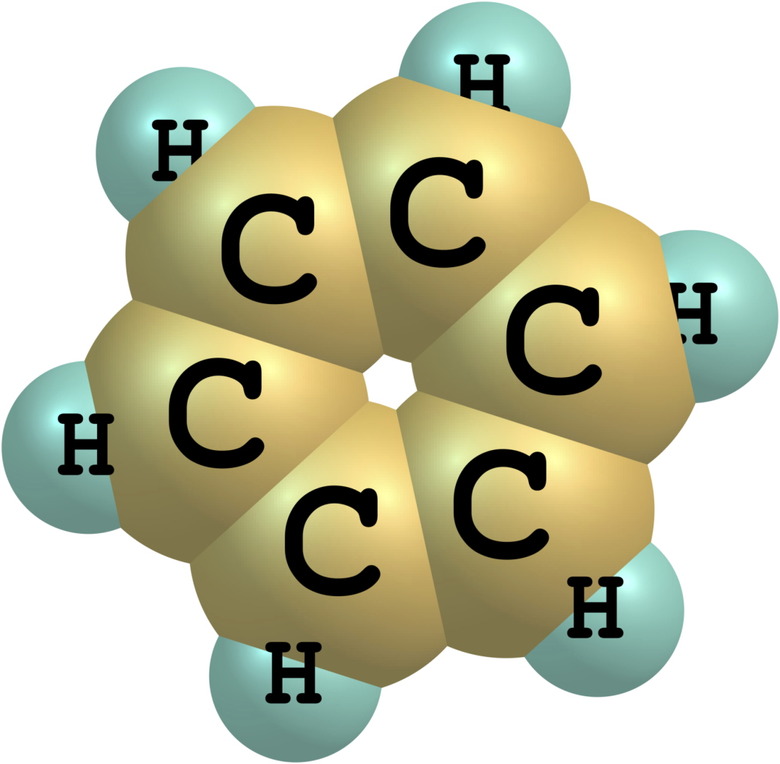
Benzene is the simplest hydrocarbon belonging to the class of organic compounds known as aromatics. Its formula, C6H6, reflects its ring structure, in which all six carbon atoms share electrons equally and the carbon-to-carbon linkages are intermediate between single and double bonds. At room temperature, benzene is a colorless liquid with the smell of 'sweet gasoline'. Benzene boils at 176.2 degrees Fahrenheit and freezes below 41.9 degrees Fahrenheit. Benzene is a dangerous chemical that is highly flammable and carcinogenic. It occurs naturally as a component of crude oil, and there are several ways to prepare it.
Cracking Crude Oil
Cracking Crude Oil
Preparing benzene from crude oil using heat is called cracking. Cracking is a multistep process in which a facility vaporizes raw petroleum, adds steam and then briefly passes the gaseous mixture through a furnace at temperatures between 1,300 and 1,650 degrees Fahrenheit. The resulting mixture of hydrocarbons is called raw pyrolysis gas. Solvents, usually alcohols, then extract benzene and other aromatic compounds, including methylbenzene. Finally, the dissolved compounds undergo fractional distillation, which separates out the different components, including benzene.
Reforming Naphtha
Reforming Naphtha
Naphtha refers to straight-chain, or aliphatic, hydrocarbons containing 5–10 carbon atoms. Naphtha is derived primarily from petroleum and natural gas. To reform naphtha into benzene, reactors must first remove any sulfurous impurities and then mix the naphtha with hydrogen at 930 degrees Fahrenheit, a process called hydroforming. The gas passes over a catalyst, such as platinum or rhenium, under 5 atmospheres of pressure. This process converts aliphatic hydrocarbons to their corresponding aromatic compounds. Benzene, formed from the six-carbon aliphatic compound hexane, and the other hydrocarbons are then dissolved and distilled to separate the different compounds.
Toluene Dispropotionation
Toluene Dispropotionation
Methylbenzene, also known as toluene, is a byproduct of naphtha reformation but is of limited commercial value. Processing plants can convert toluene into the more valuable hydrocarbons benzene and xylene. A toluene-hydrogen mixture passes over a catalyst—usually zeolite, a mineral containing aluminosilicates—under conditions of 15–25 atmospheres of pressure and 800–900 degrees Fahrenheit. Equipment then distills the resulting hydrocarbon mixture to separate the benzene, toluene and xylene fractions. The toluene is recycled for further disproportionation.
Toluene Hydrodealkylation
Toluene Hydrodealkylation
An alternative method to prepare benzene from toluene is hydrodealkylation. Reactors compress toluene and hydrogen to pressures between 20 and 60 atmospheres and heat the mixture to temperatures between 930 and 1,220 degrees Fahrenheit. In the presence of a catalyst, a reaction converts the mixture to benzene and methane. Suitable catalysts include chromium, molybdenum and platinum. The leftover hydrogen is recycled, and the benzene is separated out by distillation. This method results in a conversion rate of 90 percent.
Cite This Article
MLA
Finance, Eric Bank, MBA, MS. "How Benzene Is Made" sciencing.com, https://www.sciencing.com/make-benzene-5164625/. 24 April 2017.
APA
Finance, Eric Bank, MBA, MS. (2017, April 24). How Benzene Is Made. sciencing.com. Retrieved from https://www.sciencing.com/make-benzene-5164625/
Chicago
Finance, Eric Bank, MBA, MS. How Benzene Is Made last modified March 24, 2022. https://www.sciencing.com/make-benzene-5164625/