How To Find How Many Protons, Neutrons & Electrons Are In Isotopes
Matter comes in many different sizes, shapes and colors. Consider chlorine, a yellowish gas, or lead, a gray-black solid, or mercury, a silvery liquid. Three very different elements, each material made of only one kind of atom. The differences in matter comes down to the tiniest differences in atomic structure.
TL;DR (Too Long; Didn't Read)
Understand that isotopes of an element have different mass numbers but the same number of protons. Using the Periodic Table, find the atomic number of the element. The atomic number equals the number of protons. In a balanced atom, the number of electrons equals the number of protons. In an unbalanced atom, the number of electrons equals the number of protons plus the opposite of the ion charge. Calculate the number of neutrons by subtracting the atomic number from the mass number. If the mass number of a specific isotope isn't known, use the atomic mass from the Periodic Table, rounded to the nearest whole number, minus the atomic number to find the average number of neutrons for the element.
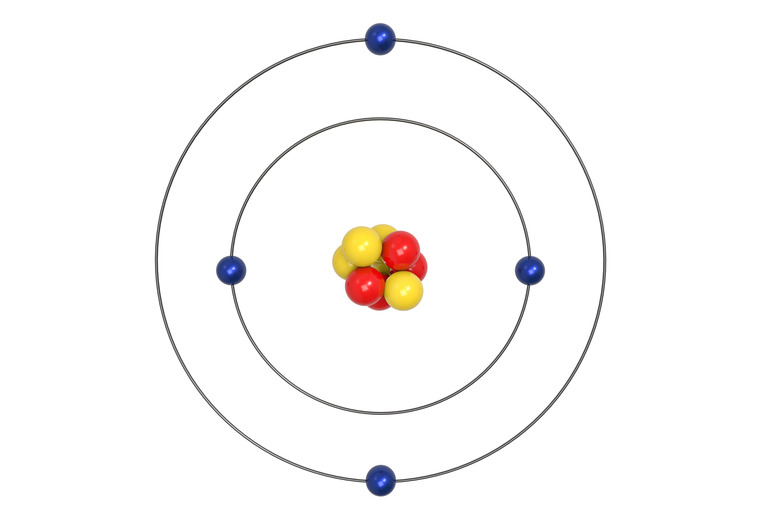
Structure of Atoms
Structure of Atoms
Three main particles form every atom. Protons and neutrons cluster in the nucleus at the center of the atom. Electrons form a spinning cloud around the nucleus. Protons and neutrons make up the mass of atoms. Electrons, miniscule compared to the protons and neutrons, contribute very little to the overall mass of atoms.
Atoms and Isotopes
Atoms and Isotopes
Atoms of the same element have the same number of protons. All copper atoms have 29 protons. All helium atoms have 2 protons. Isotopes occur when atoms of the same element have different masses. Since the number of protons of an element doesn't change, the difference in mass occurs because of different numbers of neutrons. Copper, for example, has two isotopes, copper-63 and copper-65. Copper-63 has 29 protons and a mass number of 63. Copper-65 has 29 protons and mass number 65. Helium has 2 protons and almost always has a mass number of 4. Very rarely, helium forms the isotope helium-3, which still has 2 protons but has a mass number of 3.
One method of writing the formula for an isotope shows the element name or symbol followed by the mass number, as helium-4 or He-4. Another shorthand identification of isotopes shows the mass number as a superscript and the atomic number as a subscript, both shown preceding the atomic symbol. For example, 42He indicates the helium isotope with mass number 4.
Periodic Table of Elements
Periodic Table of Elements
The arrangement of the Periodic Table of Elements provides essential information to finding the number of protons, neutrons and electrons in atoms. The modern Periodic Table puts the elements in order of their protons. The first element on the table, hydrogen, has one proton. The last element (at least for now) on the table, Oganesson or Ununoctium, has 118 protons.
How Many Protons?
How Many Protons?
The atomic number on the Periodic Table identifies the number of protons in any atom of that element. Copper, atomic number 29, has 29 protons. Finding the atomic number of an element reveals the number of protons.
How Many Neutrons?
How Many Neutrons?
The difference between isotopes of an element depends on the number of neutrons. To find the number of neutrons in an isotope, find the mass number of the isotope and the atomic number. The atomic number, or number of protons, is found on the Periodic Table. The atomic mass, also found on the Periodic Table, is the weighted average of all the isotopes of the element. If no isotope is identified, the atomic mass can be rounded to the nearest whole number and used to find the average number of neutrons.
For example, the atomic mass of mercury is 200.592. Mercury has several isotopes with mass numbers ranging from 196 to 204. Using the average atomic mass, calculate the average number of neutrons by first rounding the atomic mass from 200.592 to 201. Now, subtract the number of protons, 80, from the atomic mass, 201-80, to find the average number of neutrons, 121.
If the mass number of an isotope is known, the actual number of neutrons can be calculated. Use the same formula, mass number minus atomic number, to calculate the number of neutrons. In the case of mercury, the most common isotope is mercury-202. Use the equation, 202-80=122, to find that mercury-202 has 122 neutrons.
How Many Electrons?
How Many Electrons?
A neutral isotope has no charge, meaning that the positive and negative charges balance in a neutral isotope. In a neutral isotope, the number of electrons equals the number of protons. Like finding the number of protons, finding the number of electrons in a neutral isotope requires finding the atomic number of the element.
In an ion, an isotope with a positive or negative charge, the number of protons doesn't equal the number of electrons. If protons outnumber electrons, the isotope has more positive charges than negative charges. In other words, the number of protons exceeds the number of electron by the same number as the positive charge. If the number of electrons exceeds the number of protons, the ion charge will be negative. To find the number of electrons, add the opposite of the charge imbalance to the number of protons.
For example, if an isotope has a -3 charge, as with phosphorus (atomic number 15), then the number of electrons is three greater than the number of protons. Calculating the number of electrons then becomes 15+(-1)(-3) or 15+3=18, or 18 electrons. If an isotope has a +2 charge, as with strontium (atomic number 38), then the number of electrons is two less than the number of protons. In this case, the calculation becomes 38+(-1)(+2)=38-2=36, so the ion has 36 electrons. The usual shorthand for ions shows the charge imbalance as a superscript following the atomic symbol. In the phosphorus example, the ion would be written as P-3.
Cite This Article
MLA
Blaettler, Karen G. "How To Find How Many Protons, Neutrons & Electrons Are In Isotopes" sciencing.com, https://www.sciencing.com/many-protons-neutrons-electrons-isotopes-8653077/. 10 May 2018.
APA
Blaettler, Karen G. (2018, May 10). How To Find How Many Protons, Neutrons & Electrons Are In Isotopes. sciencing.com. Retrieved from https://www.sciencing.com/many-protons-neutrons-electrons-isotopes-8653077/
Chicago
Blaettler, Karen G. How To Find How Many Protons, Neutrons & Electrons Are In Isotopes last modified March 24, 2022. https://www.sciencing.com/many-protons-neutrons-electrons-isotopes-8653077/