Which Of The Metalloids Has The Smallest Atomic Radius?
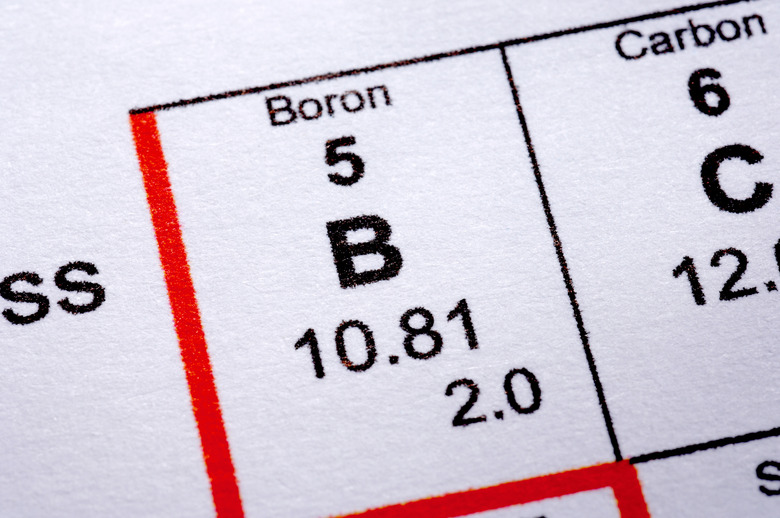
The metalloids are elements that display some properties of both metals and nonmetals. The exact list of metalloids is not agreed upon. However, boron, silicon, germanium, arsenic, antimony and tellurium are all frequently classified as metalloids. Boron has the smallest atomic radius of these metalloids.
Periodic Trends in Atomic Radius
Periodic Trends in Atomic Radius
Atomic radius increases as you move vertically down the periodic table. As you move down a group of the periodic table, each new row indicates the addition of an energy level. This increases the average distance of the outermost electron from the nucleus. However, atomic radius decreases as you move from left to right across a period of the periodic table. The protons and electrons in an atom increase, but the electrons fill valence shells in the same energy level. The electron cloud does not grow substantially in size, but the net charge of the nucleus does. Hence, the electrons are pulled closer to the nucleus and the atomic radius decreases.
Cite This Article
MLA
Murmson, Serm. "Which Of The Metalloids Has The Smallest Atomic Radius?" sciencing.com, https://www.sciencing.com/metalloids-smallest-atomic-radius-12377/. 24 April 2017.
APA
Murmson, Serm. (2017, April 24). Which Of The Metalloids Has The Smallest Atomic Radius?. sciencing.com. Retrieved from https://www.sciencing.com/metalloids-smallest-atomic-radius-12377/
Chicago
Murmson, Serm. Which Of The Metalloids Has The Smallest Atomic Radius? last modified March 24, 2022. https://www.sciencing.com/metalloids-smallest-atomic-radius-12377/