Parts Of The Periodic Table
The periodic table is a graphical layout of the chemical elements, organized into rows and columns according to their basic characteristics. The table allows scientists to easily grasp the relationships and similarities among the elements, which are the building blocks of all matter.
The Elements of the Periodic Table
The Elements of the Periodic Table
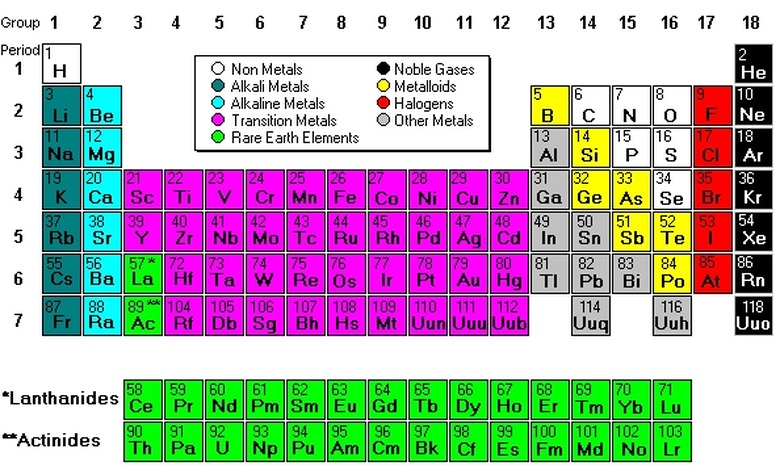
At the time of publication, the periodic table consists of 118 elements, with 94 of these naturally occurring on the Earth and the rest being synthetic. Each element is contained in a small block. Information in the block includes the name of the element, its chemical symbol, atomic number and atomic mass.
Atomic Number and Mass
Atomic Number and Mass
As the table progresses from left to right, and top to bottom, the atomic number of the elements increases. The atomic number is the count of the protons in the atomic nucleus. The table also shows atomic mass, which is the total number of neutrons and protons in the atom's nucleus, averaged according to the relative abundance of the element's isotopes. For elements with no stable isotope, the table gives in parenthesis the atomic mass of the isotope with the longest half-life; in other words, the most stable form of the element.
The Periods
The Periods
The seven rows in the table represent periods. Each element in a single row has the same number of electron shells that surround the atomic nucleus. The elements hydrogen and helium have a single orbital shell; elements in the second row have two orbitals, and so on. In the seventh period, elements have a seventh orbital shell.
The Groups
The Groups
The table's 18 columns, read vertically from top to bottom, represent groups. All elements in a group have the same number of electrons orbiting the nucleus in the outermost shell. The exceptions to this rule include hydrogen, helium and the "transitional elements," which occupy groups three through 12. Elements in a group share important chemical characteristics. Group 18, for example, includes the "inert" or "noble" gases. Group 17 includes the five halogens.
Graphic Indicators
Graphic Indicators
Some periodic tables display a color code that shows the state of the element–solid, liquid, gas, or unknown–at zero degrees Centigrade. Borders may show whether the element is naturally occurring (solid border), occurring only as a result of radioactive decay (dashed border) or artificial (dotted border). A single thick line sometimes appears in the periodic table dividing the elements into metallic (to the left) and non-metallic (to the right).
Lanthanides and Actinides
Lanthanides and Actinides
At the bottom of the periodic table are two additional rows of 14 elements each. The top row shows the lanthinides, elements 58 through 71; these are also called rare earths. The bottom row is the actinides, which begin with element 90 and end at 103; note, however, that elements beyond 103 exist and will continue to be added to the periodic table as scientists discover new ones. The first elements in these two series are contained in the main body of the periodic table: lanthanum (57) and actinium (89).
The Element Groups
The Element Groups
There are nine basic groups of elements shown in the periodic table. They are the alkali metals, alkaline earth metals, transition metals, other metals, metalloids, non-metals, halogens, noble gases and rare earth elements.
Cite This Article
MLA
Streissguth, Tom. "Parts Of The Periodic Table" sciencing.com, https://www.sciencing.com/parts-periodic-table-5414878/. 24 April 2017.
APA
Streissguth, Tom. (2017, April 24). Parts Of The Periodic Table. sciencing.com. Retrieved from https://www.sciencing.com/parts-periodic-table-5414878/
Chicago
Streissguth, Tom. Parts Of The Periodic Table last modified March 24, 2022. https://www.sciencing.com/parts-periodic-table-5414878/