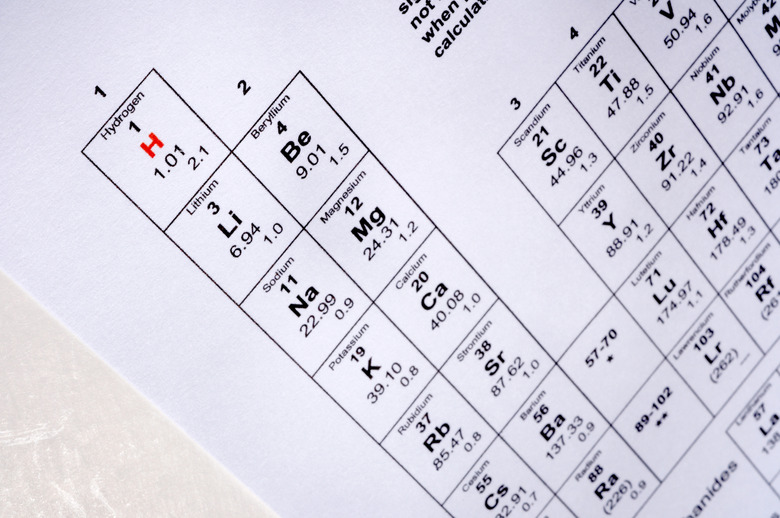
Why Is The Periodic Table Arranged In Columns & Rows?
The elements of the periodic table are arranged by increasing atomic number. These elements are then wrapped into rows and columns corresponding to the properties of the elements in each row and column.
Atomic Number
Atomic Number
Each element has a unique atomic number determined by the number of protons in the nucleus. For example, the atomic number of carbon (C) is 6, since all carbon atoms have six protons.
Neutral Atoms
Neutral Atoms
In a neutral atom, the number of electrons is equal to the number of protons. As an example, a neutral atom of carbon has six electrons and six protons.
Electron Configuration
Electron Configuration
Electrons fill energy shells from lowest energy to highest energy. The electrons in the outermost shell of the atom are termed valence electrons and are the electrons involved in chemical bonding.
Periods on the Periodic Table
Periods on the Periodic Table
The rows on the periodic table are called periods. All the elements in a period have valence electrons in the same shell. The number of valence electrons increases from left to right in the period. When the shell is full, a new row is started and the process repeats.
Groups on the Periodic Table
Groups on the Periodic Table
Atoms with a similar number of valence electrons tend to have similar chemical properties. This correlation appears in columns (known as families) on the periodic table. For example, the alkaline earth family (Group 2) all have two valence electrons and share similar chemical properties.
Cite This Article
MLA
Chandler, David. "Why Is The Periodic Table Arranged In Columns & Rows?" sciencing.com, https://www.sciencing.com/periodic-table-arranged-columns-rows-5801870/. 24 April 2017.
APA
Chandler, David. (2017, April 24). Why Is The Periodic Table Arranged In Columns & Rows?. sciencing.com. Retrieved from https://www.sciencing.com/periodic-table-arranged-columns-rows-5801870/
Chicago
Chandler, David. Why Is The Periodic Table Arranged In Columns & Rows? last modified March 24, 2022. https://www.sciencing.com/periodic-table-arranged-columns-rows-5801870/