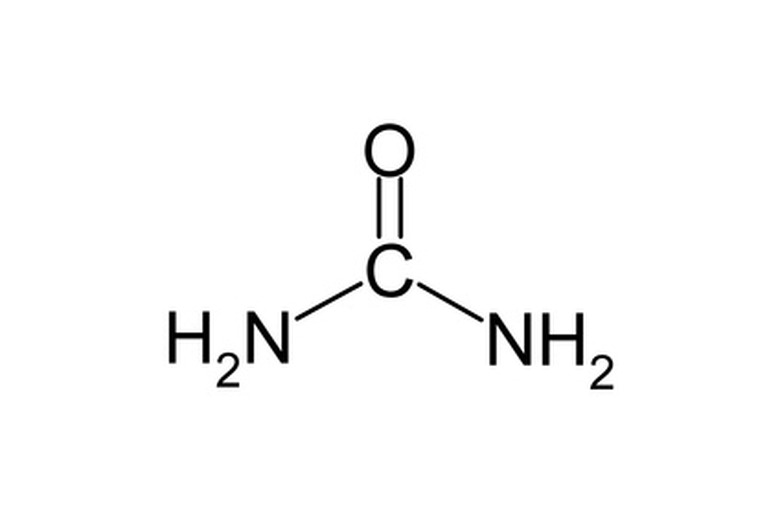
Physical Properties Of Urea
Urea is a mineral that is only stable in an arid environment. It is named after the Greek word "oura" meaning urine and that's exactly what it is. Although it is not hazardous, use precautions as necessary when handling urea.
Physical Appearance
Physical Appearance
Urea appears to be a light brown or light yellow. It is normally translucent and comes in the form of a liquid or solid (pellets).
Odor
Odor
The smell of urea is almost non-existent. If a sample of urea is not odorless, it will have a slight ammonia scent.
Density
Density
The density of this mineral is 1.33 g/cm3. Density is the ratio between the mass and volume.
Specific Gravity
Specific Gravity
The specific gravity of urea is 1.34 at room temperature: 68 degrees Fahrenheit or 20 degrees Celsius. This makes the mineral heavier than water.
Solubility
Solubility
Urea is soluble in water. Its solubility ratio is 119 grams per 100 grams water at a temperature of 77 degrees Fahrenheit or 25 degrees Celsius.
Molecular Weight
Molecular Weight
The molecular weight, or molar mass, of urea is measured at 60.06 grams. This measurement indicates the mass of one mole of urea.
Decomposition
Decomposition
Urea decomposes at 270.8 degrees Fahrenheit (132.7 degrees Celsius); it decomposes into ammonia and carbon dioxide. If burned, it emits small amounts of nitrogen oxides.
Cite This Article
MLA
Glass, Vanessa. "Physical Properties Of Urea" sciencing.com, https://www.sciencing.com/physical-properties-urea-6369247/. 24 April 2017.
APA
Glass, Vanessa. (2017, April 24). Physical Properties Of Urea. sciencing.com. Retrieved from https://www.sciencing.com/physical-properties-urea-6369247/
Chicago
Glass, Vanessa. Physical Properties Of Urea last modified March 24, 2022. https://www.sciencing.com/physical-properties-urea-6369247/