Purpose Of Titration
The purpose of titration is to determine an unknown concentration in a sample using an analytical method. Titration requires three basic components: a liquid of known molarity or normality, called the titrant, the sample or liquid in need of measuring, called the titrand, and a calibrated device for dispensing the titrant drop by drop into the titrand. When the titration reaches an endpoint, the amount of titrant is recorded and used to calculate the unknown concentration.
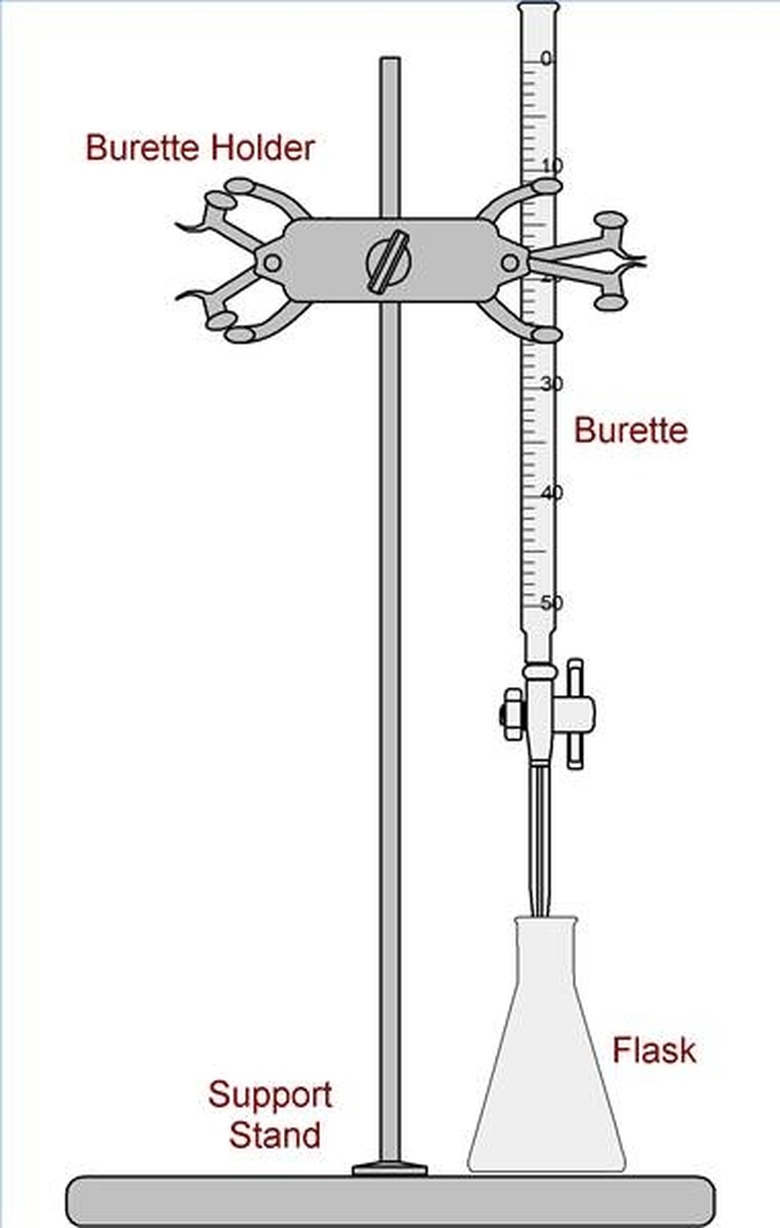
A Titration Illustration
A Titration Illustration
A chemist is testing for the amount of chloride in a waste water sample. The method used is an easy titration to perform. The chemist places a calibrated burette on a burette stand. She then measures an aliquot of sample in a 100mL Erlynmeyer flask, in this case 50mL of liquid. All measurements are documented on a piece of paper or in a log book. The chemist fills the burette with a known concentration of mercuric nitrate solution, the solution needed for this particular chloride test. She then places five drops of indicator solution in the sample and acidifies it with nitric acid. It turns yellow. The chemist documents the beginning level of solution in the burette. She then places the sample under the burette and slowly, drop by drop, lets the titrant fall into the titrand, or sample, until a purple endpoint is reached. The chemist documents how much titrant was used and calculates the value of chloride in the solution using a simple equation specified by the method.
Types of Titrations
Types of Titrations
The titration mentioned above is a complexation titration. The endpoint color is exhibited when the indicator solution forms a complex with the excess mercuric ions from the titrant. This happens between a pH of 2.3 and 2.8. The most common types of titrations are acid/base titrations. They are used for quantifying analytes, the unknown ion or compound being tested for, in addition to being used to standardize acids and bases. Acid/base titrations sometimes require the use of a pH meter, while other times the method calls for an indicator solution, like in the example above. Another type of titration is a redox reaction, when combining the titrant and titrand causes a gain in electrons. This gain is called a reduction.
About the Burette
About the Burette
The calibrated burette is the main piece of equipment required for a titration method. Calibration is important because it is essential for the burette to be as accurate as possible in order to dispense very precise amounts of liquid into the sample. A burette is a long cylindrical piece of glass with an open top for pouring, or pumping, in the titrant. At the bottom there is a carefully formed tip for dispensing. Burettes usually have a plastic stopper that can easily be turned to deliver mere fractions of a drop of titrant, if needed. Burettes come in many sizes and are marked in milliliters and fractions of milliliters.
Other Possible Instrumentation
Other Possible Instrumentation
Burettes are the most common piece of instrumentation used in titrations, but electronic devices can also be used. Potentiometric titrations can use a calibrated pH meter to determine an endpoint. The endpoint pH reading is similar to using an indicator solution except the chemist is using an instrument to find the exact potential rather than a color change. Complexation titrations can use an ion selective electrode to determine at what point a complex has been reached. Spectrometry is another option; it allows the chemist to determine very small color changes in the titrand.
Indicator Solutions
Indicator Solutions
Indicator solutions are not always necessary for titrations, but they can make manual titrations with a burette easier. One of the most common indicator solutions used in acid/base titrations is the phenolphthalein indicator. This indicator changes to a bright pink when the pH is adjusted to 8.3. This works because the phenolphthalein molecule is colorless but its ion is colored. As the solution becomes more basic the molecule looses its H+ ions and the ionized phenolphthalein gives off its characteristic pink color. When ionization is complete the indicator solution has turned the entire sample pink, producing the experiment's endpoint.
Cite This Article
MLA
Bartleson, Becca. "Purpose Of Titration" sciencing.com, https://www.sciencing.com/purpose-titration-5406434/. 24 April 2017.
APA
Bartleson, Becca. (2017, April 24). Purpose Of Titration. sciencing.com. Retrieved from https://www.sciencing.com/purpose-titration-5406434/
Chicago
Bartleson, Becca. Purpose Of Titration last modified August 30, 2022. https://www.sciencing.com/purpose-titration-5406434/