How To Find A Quantum Number
Each element has a set of four quantum numbers that describes the energy, shape, orientation in space and spin of its electrons. These numbers are found by solving Schroedinger's equation and solving them for specific wave functions, also known as atomic orbitals. There is an easy way to find the individual quantum numbers for elements simply by using the periodic table. The table is set up like a grid, with the vertical being periods and the horizontal the groups. Quantum numbers are found using the periods of the chart.
Step 1
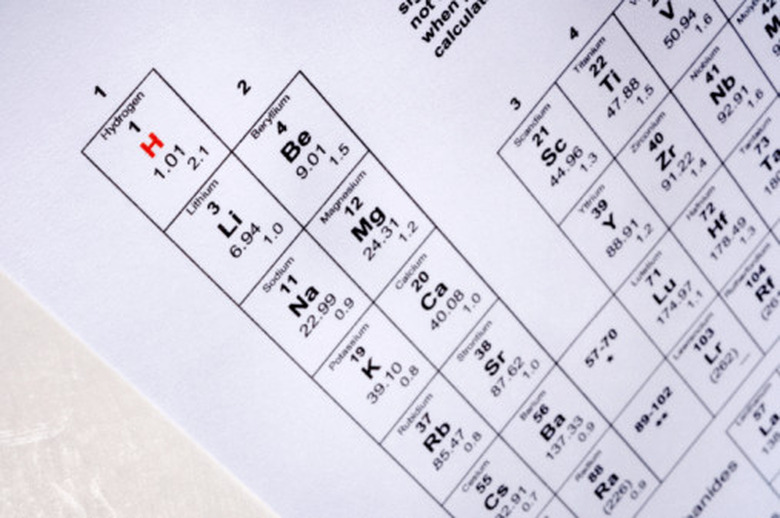
Look at the Periodic Table of Elements and find the element that you want to know the quantum number for. Find the principal number, which denotes the element's energy, by looking in which period the element is found. For example, sodium is in the third period of the table, so its principal quantum number is 3.
Step 2
Denote the principal quantum number as n and the second number, shape, is anywhere from 0 to n-1. So for sodium, the second quantum numbers are 0,1 and 2. Since this number represents the shape of a single electron in the orbital, the second quantum number for the element can include 0,1 and 2 depending on the electron in question.
Step 3
Call the second quantum number l. Represent the magnetic quantum number that denotes orientation of the electron in space by -l to +l. For for the case of sodium, that could be -2, -1, 0,1 and 2, if the second quantum number was 2.
Step 4
Consider the rotation of the electron like a clock. The only directions they can rotate is clockwise or counterclockwise, represented by -1/2 or +1/2. These are the only values available for the fourth quantum number.
TL;DR (Too Long; Didn't Read)
The Pauli Exclusion Principle states that no two electrons within an element can have the same quantum number. Every variation of the possible quantum numbers are represented.
Cite This Article
MLA
Cooper, Brock. "How To Find A Quantum Number" sciencing.com, https://www.sciencing.com/quantum-number-8262031/. 24 April 2017.
APA
Cooper, Brock. (2017, April 24). How To Find A Quantum Number. sciencing.com. Retrieved from https://www.sciencing.com/quantum-number-8262031/
Chicago
Cooper, Brock. How To Find A Quantum Number last modified August 30, 2022. https://www.sciencing.com/quantum-number-8262031/