How To Read The Periodic Table For Kids
You may rarely think about it, but literally everything around you is made up of elements found on the periodic table of elements. From humans to trees to the unseen air, all matter is comprised of the elements found on that chart with all the letters. Understanding the periodic table is not difficult, if you understand why each element is in its place. It is important for kids to understand how to read it. There is a method to the arrangement and here is a good way to teach it.
Step 1
Ask the child to look at one of the squares on the periodic table. It will show a number at the top, two or three letters and a number at the bottom. The number at the top is the atomic number. It tells the number of protons in an element. The two letters are the atomic symbol. This is the abbreviated form of the element's name or its Latin name. The bottom number is the atomic mass. It tells how much one atom of the elements weighs in atomic mass units.
Step 2
Ask the child to count the number of columns in the table. There are 18 columns. The columns are called groups . The groups are based on atomic structure of the elements; all elements in a group have similar atomic structure.
Step 3
Ask the child to count the number of rows in the table. There are 7 rows (9 counting the two separated rows). The rows are called periods. The periods are based on the chemical properties; elements belonging to the same period have similar properties, such as volatility and conductivity.
Step 4
Explain the categories of the elements. Some tables have the elements arranged in color-coded areas. This color coding distinguishes the categories of the elements. The first column is made up of elements known as alkali metals. The second column elements are called alkaline earth metals. The transition metals include 38 elements from columns three through 12. Seven metals from groups 13 through 15 are called simply other metals. The metalloids are eight elements from groups 13 through 16. The six remaining elements from groups 13 through 16 are the other nonmetals. Column 17 makes up the halogens. Column 18 is comprised of the noble gases.
Step 5
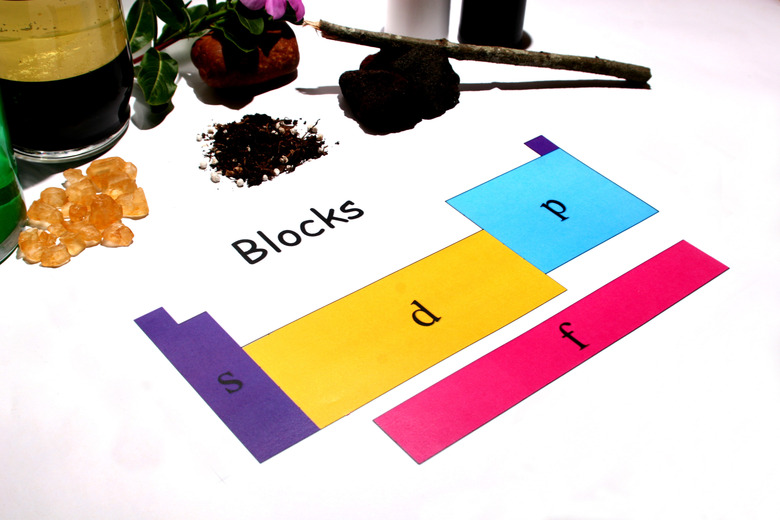
Explain the blocks. The periodic table of elements can also be separated into blocks. There are four major blocks of elements: s-block, d-block, p-block and f-block. These blocks represent how the elements differ in terms of the location of their last electron. Electrons of an atom are separated into orbitals. The s-block (first two groups) have electrons in the s-orbital. The d-block (groups 3 through 12) have electrons in the d-orbital. The p-block (groups 13 through 18) have electrons in the p-orbital. The f-block is made up of the two separate rows.
Step 6
Explain the two separate rows. Usually, a periodic table will show two rows separate from the main table. These are the rare earth metals. The top row elements are called lanthanides and the bottom row elements are called actinides. Most of these are either man-made or found in very small quantities on Earth.
Cite This Article
MLA
Cato, Jeremy. "How To Read The Periodic Table For Kids" sciencing.com, https://www.sciencing.com/read-periodic-table-kids-6326359/. 24 April 2017.
APA
Cato, Jeremy. (2017, April 24). How To Read The Periodic Table For Kids. sciencing.com. Retrieved from https://www.sciencing.com/read-periodic-table-kids-6326359/
Chicago
Cato, Jeremy. How To Read The Periodic Table For Kids last modified March 24, 2022. https://www.sciencing.com/read-periodic-table-kids-6326359/