How To Test For Ethanol Content
Ethanol is one of the most widely used of all industrial solvents. For this reason, it can commonly be found in a whole range of home based products from medicine to beer, detergents to the fuel in our vehicles. The concentration of ethanol selected will vary with the purpose for which it is used, because the result can either be useful or dangerous to our health. For this reason, it is important to devise a method to test a substance in order to determine ethanol content beyond a doubt. Read on to learn how to test for ethanol.
Step 1
Observe whether or not the liquid that you are testing is clear and colorless. Smell the liquid too, so as to confirm that it has the pleasant odor that is a typical characteristic of Ethanol. While this visual and smell test is not exact, it is a very quick and easy first step.
Step 2
Determine the sample liquid's specific gravity. The specific gravity (SG) of a solid or liquid substance is simply a ratio of its density to the density of water at a specific temperature. Every substance has a unique specific gravity, and ethanol's specific gravity is 0.815 at 68 degrees F.
Step 3
Ensure that the your hygrometer is calibrated so as to ensure accurate results. Also ensure that the cylinder and thermometer are both free of impurity, which would contaminate your ethanol.
Step 4
Use the thermometer to make sure that the temperature of the sample you are testing is 68 degrees F.
Step 5
Pour 100 millimeters of your test sample into the measuring cylinder, and let it stand for a while.
Step 6
Immerse the clean, dry hydrometer into the test sample. Ensure that it is immersed up to at least three quarters into the ethanol.
Step 7
Allow it to settle an then take your reading. If the reading comes within the 0.815 range, this indicates that the substance is ethanol.
Things Needed
- Hygrometer
- 100 ml measuring cylinder
- Thermometer
TL;DR (Too Long; Didn't Read)
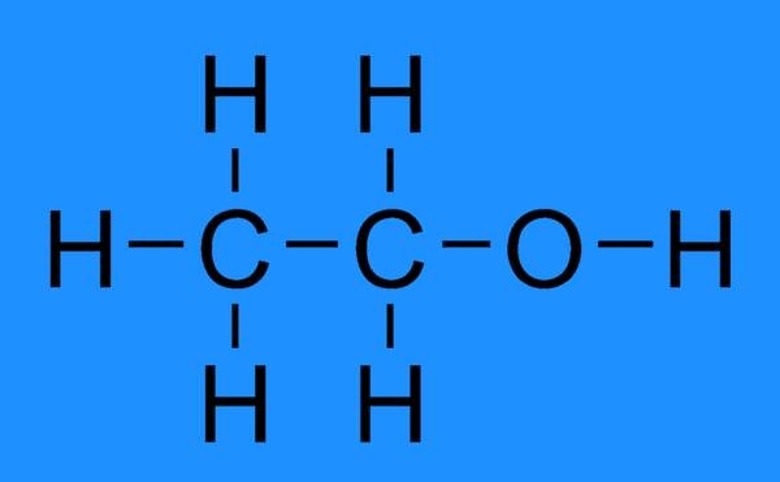
Ethanol is categorized as a straight chain chemical compound, which belongs to the broad group of alcohols. Its chemical or empirical formula is C2H6O, indicating that it is an isomer of dimethyl ether.
Warning
It is essential that you do not contaminate your ethanol sample or your apparatus. Any contamination will result in false readings.
Cite This Article
MLA
Contributor, . "How To Test For Ethanol Content" sciencing.com, https://www.sciencing.com/test-ethanol-content-4598588/. 24 April 2017.
APA
Contributor, . (2017, April 24). How To Test For Ethanol Content. sciencing.com. Retrieved from https://www.sciencing.com/test-ethanol-content-4598588/
Chicago
Contributor, . How To Test For Ethanol Content last modified March 24, 2022. https://www.sciencing.com/test-ethanol-content-4598588/