Thermal Energy: Definition, Equation, Types (W/ Diagram & Examples)
Thermal energy, also called heat energy or simply heat, is a type of internal energy an object is said to possess owing to the kinetic energy of its constituent particles.
Energy itself, while easy enough to define in mathematical terms, is among the more elusive quantities in physics in terms of what it fundamentally is. There are many forms of energy, and it is easier to define energy in terms of the limits on its arithmetic behavior than it is to frame it in precise language.
Unlike translational or rotational kinetic energy, which arise from motion across some linear distance or in a circle, respectively (and these can occur together, as with a thrown Frisbee), heat energy comes from the motion of vast numbers of tiny particles, movement that can be thought of as vibration around fixed points in space.
On average, each particle is found in a particular place within the extended system as it wanders frantically about that point, even if at no point in time is the particle statistically likely to be found there. This is rather like the average position of the Earth over time being close to the center of the sun even though this arrangement (fortunately!) never occurs.
Any time two materials come in contact, including air, friction results, and some of the total energy of the system – which, as you'll see, must always remain constant – is transformed into thermal energy.
The object and its surroundings experience an increase in temperature, which is the quantifiable manifestation of thermal energy and heat transfer, measured in degrees Celsius (°C), degrees Fahrenheit (°F) or Kelvin (K). When objects lose heat, they drop to a lower temperature.
Just What Is Energy?
Just What Is Energy?
Energy comes in various forms as well as various units, the most common being the **joule (J)**, named for James Prescott Joule. The joule itself has units of force times distance, or newton-meters (N⋅m). More fundamentally, the units of energy are kg⋅m2/s2.
One concept closely tied to energy is work, which has units of energy but is not regarded as energy by physicists. Work can be said to be "done on" a system by adding energy to it, which results in a physical change to the system (e.g., it moves a piston or rotates a magnetic coil – that is, does useful work). A system is any physical set-up with clearly defined boundaries, which can even be the Earth as a whole.
In addition to heat energy (usually written Q) and kinetic energy (the "normal" linear or rotational sort), other types of energy include potential energy, mechanical energy and electrical energy. The critical aspect of energy is that, no matter how it appears in any system, it is always conserved.
Thermal Energy: The Least Useful Form of Energy
Thermal Energy: The Least Useful Form of Energy
When there is transfer of thermal energy to the environment (i.e., it "dissipates" or "is lost"), of course no energy is actually being destroyed in any way, as this would violate the conservation of energy.
This heat, however, cannot be fully recaptured and reused, which is why it is called a less useful form of energy. Whenever you pass a building or ground vent in the winter and an endless cloud of steam or warm air is flowing out, that is a clear example of thermal energy that is "useless" energy. On the other hand, a heat engine like the one in gasoline-powered cars uses thermal energy for mechanical energy.
Heat Energy and Temperature
Heat Energy and Temperature
The temperature of an object or system is a measure of the average translational kinetic energy per molecule of that object, while thermal energy is the total internal energy of the system. When particles move, there is always kinetic energy. Moving heat upward against a temperature gradient requires work, such as the use of heat pumps.
Heat and the Everyday World
Heat and the Everyday World
Thermal energy may be appearing here as a rogue quantity, but it can be and is put to excellent use in cooking and other realms. When you digest food, you convert chemical energy from the bonds in carbohydrates, proteins and fat to heat ("calories" instead of joules in common terms).
Friction generates heat, often in a hurry. If you rub your hands together quickly, they will warm up fast. An automatic weapon fires bullets out of the barrel so quickly that the metal becomes dangerously hot to the touch almost immediately.
Thermal Energy and the Conservation of Energy: Example
Thermal Energy and the Conservation of Energy: Example
Consider a marble rolling around inside a bowl. The "system" also includes the environment (i.e., the Earth as a whole). As it moves up the side, more of its total energy converted to gravitational potential energy; as it speeds up near the bottom, more of that energy is transformed to kinetic energy. If this were the whole story, the marble would keep going up and down forever, reaching the same heights and speeds with each cycle.
Instead, each time the marble comes up the side, it climbs a little less high, and its velocity at the bottom is a bit less, until eventually the marble comes to rest at the bottom. This is because the whole time the marble was rolling, more and more of the total-energy "pie" was being converted to a larger and larger "slice" of thermal energy and dissipated into the environment, no longer usable by the marble. At the bottom, all the system's energy has "become" thermal energy.
Thermal Energy Equation: Heat Capacity
Thermal Energy Equation: Heat Capacity
One of the equations you may encounter is the one for heat capacity:
\(Q=mC\Delta T\)
where Q is thermal energy in joules, m is the mass of the object being heated, C is the object's specific heat capacity and delta T is its change in temperature in Celsius. The specific heat capacity of a substance is the amount of energy required to raise the temperature of 1 gram of that substance by 1 degree Celsius.
Higher heat capacities thus imply a greater resistance to temperature change for a given mass of a substance, and more mass by itself means a higher heat capacity. This makes intuitive sense; if you exposed 10 mL of water to "high" in a microwave for one minute, the temperature change will be far greater than if you heated 1,000 mL of water starting at the same temperature for the same length of time.
The Laws of Thermodynamics
The Laws of Thermodynamics
Thermodynamics is the study of how work, heat and internal energy interact in a system. Importantly, it is concerned only with large-scale observations that can be measured; the kinetic theory of gases addresses vibrational-level interactions.
The first law of thermodynamics states that changes in internal energy can be accounted for by heat losses: ΔE = Q – W, where ΔE is change in internal energy (Δ is the Greek letter "delta," and means "difference" here), Q is the amount of thermal energy transferred into the system and W is the work done by the system on the surroundings.
The second law of thermodynamics states that whenever work is done, the amount of entropy in the atmosphere increases. Thus the flow of thermal energy is continually causing entropy to increase.
- Entropy (S) is a state variable, a thermodynamic property of a system that loosely means "disorder," and its movement can be expressed as
\(\Delta S=\frac{\Delta Q}{T}\)
The third law of thermodynamics states that the entropy S of a system approaches a constant value as the temperature T nears absolute zero (0 K, or -273 C).
When one object is at a higher temperature than a nearby object, this temperature difference favors energy transfer in the form of heat to the cooler object.
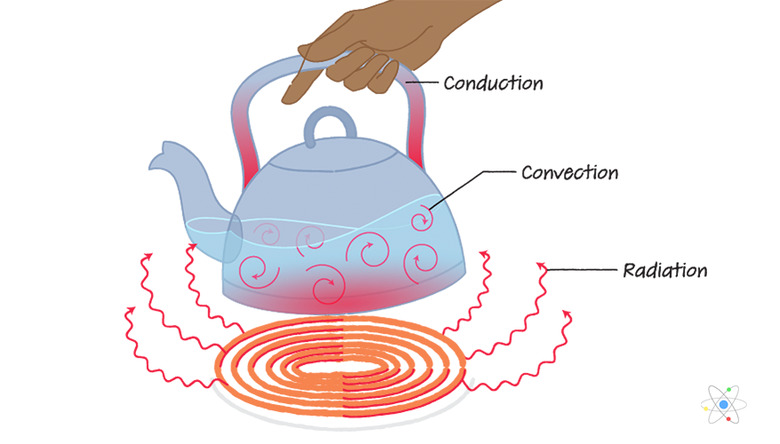
There are three basic ways to bring about the transfer of heat from one object to another: Conduction (direct contact), convection (movement through a liquid or gas) and thermal radiation (movement through space).
Cite This Article
MLA
Beck, Kevin. "Thermal Energy: Definition, Equation, Types (W/ Diagram & Examples)" sciencing.com, https://www.sciencing.com/thermal-energy-definition-equation-types-w-diagram-examples-13720809/. 28 December 2020.
APA
Beck, Kevin. (2020, December 28). Thermal Energy: Definition, Equation, Types (W/ Diagram & Examples). sciencing.com. Retrieved from https://www.sciencing.com/thermal-energy-definition-equation-types-w-diagram-examples-13720809/
Chicago
Beck, Kevin. Thermal Energy: Definition, Equation, Types (W/ Diagram & Examples) last modified August 30, 2022. https://www.sciencing.com/thermal-energy-definition-equation-types-w-diagram-examples-13720809/