What Does Titration Mean?
Titration is a common analytical method in chemistry laboratories. Most students have to do at least one acid-base titration in the lab before they graduate, and phenolphthalein is usually the indicator they use. Although it may sometimes feel like a tedious task, this procedure can reveal the concentration of unknown solutions and has multiple uses in the real world.
TL;DR (Too Long; Didn't Read)
Titration is a process that requires adding one solution to a second solution, which has an unknown concentration, until the reaction neutralizes. It shows you the concentration of the unknown solution.
Titration in the Lab
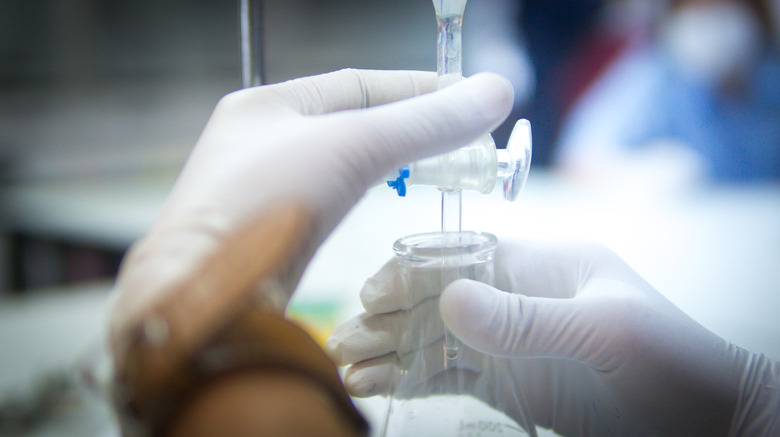
Titration in the Lab
Titration can help you discover the concentration of an unknown substance in the lab. During this process, the analyte is the unknown you want to find while the titrant or standard solution has a known concentration. You slowly add the titrant to the analyte with a buret until you reach the end point, which means neutralization. Usually, an indicator, such as phenolphthalein, changes color at the end point, so you know the process is over. For example, in an acid-base titration, phenolphthalein will change the solution color from clear to pink.
A common titration experiment in many chemistry labs uses hydrochloric acid, or HCl as the acid and sodium hydroxide, NaOH, as the base. Phenolphthalein is the indicator. You know the concentration of the base, but the amount for the acid is unknown. You can use a volumetric buret to add the base one drop at a time to the acid until the end point, which should make the beaker of acid light pink. You record the amount of base used from the volumetric buret and calculate the concentration of the acid.
Titration in Real Life
Titration in Real Life
Multiple uses for titration occur in the real world. It is often part of soil sample analysis and can help determine the amount of dissolved oxygen in water. Other uses for titration include finding certain substances in blood or urine.
Titration also serves as an important analytical tool in the food industry. It can help investigators find chemicals in food. It can also determine how much fat or water is in a product. In addition, titration can show you the vitamins in food.
Titration of Drugs
Titration of Drugs
The pharmaceutical industry uses titration for drugs. Your doctor may titrate medications to determine the right dosage. Some drugs have a narrow range where they are safe, so titration makes it possible to determine the right amount and limit side effects. Diabetic patients also rely on titration to determine how much glucose is in their blood and how much insulin they should take. They use glucose meters to measure the levels.
References
Cite This Article
MLA
Bandoim, Lana. "What Does Titration Mean?" sciencing.com, https://www.sciencing.com/what-does-titration-mean-13710466/. 27 March 2018.
APA
Bandoim, Lana. (2018, March 27). What Does Titration Mean?. sciencing.com. Retrieved from https://www.sciencing.com/what-does-titration-mean-13710466/
Chicago
Bandoim, Lana. What Does Titration Mean? last modified August 30, 2022. https://www.sciencing.com/what-does-titration-mean-13710466/