What Is Atomic Number?
In the planetary model of atomic structure, an atom consists of a heavy, positively charged nucleus surrounded by a cloud of much lighter, negatively charged electrons. Protons supply the positive charge, and each element has a different number of them. The number of protons in the nucleus determines the atomic number of an element. It's different from atomic mass or atomic weight, which take into account the presence of neutrons. Every atom of a given element always has the same atomic number, but atomic mass can vary according to the number of neutrons in the nucleus.
TL;DR (Too Long; Didn't Read)
Atomic number is the number of protons in the nucleus of an element. It defines the position of the element in the periodic table. Atomic weight, which is a another number that appears next to the element's symbol, is an average of the atomic masses of all the isotopes of that element.
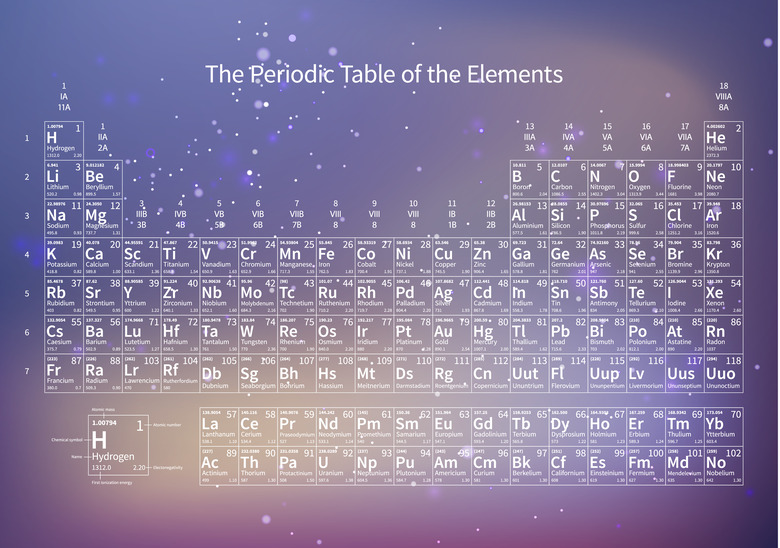
The Periodic Table
The Periodic Table
The periodic table is a chart that lists all the elements in order according to increasing atomic number. Scientists know of 118 elements. Number 118, oganesson (Og), which is an artificially produced radioactive element, was added in 2015. Oganesson has the highest atomic number because it has the highest number of protons in its nucleus. Hydrogen (H), on the other hand, has only one proton in its nucleus, so its atomic number is 1, and it appears at the beginning of the periodic table. The atomic number of each element, which is the number of protons in its nucleus, appears next to its symbol in the table. If the atomic number wasn't there, you could still tell how many protons were in the nucleus of a given element by counting the number of places between that element and hydrogen.
Atomic Number Is Not Atomic Mass or Atomic Weight
Atomic Number Is Not Atomic Mass or Atomic Weight
If you look up an element in the periodic table, you'll see another number next to its atomic number. This is the atomic weight of the element, and it's usually twice the atomic number or more. Atomic weight isn't the same as atomic mass.
The atomic mass of an atom is the mass of all the protons and neutrons in the nucleus. Electrons have such small masses compared to nucleons that they are considered negligible. Atomic mass is expressed in atomic mass units (amu) for a single atom and in grams per mole for macroscopic quantities. A mole is quantified as Avogadro's number (6.02 × 1023) of atoms.
An atom of a given element always has the same number of protons. If it had a different number, it would be a different element. However, atoms of the same element can have different numbers of neutrons. Each version is called an isotope of that element, and each isotope has a different atomic mass. The atomic mass listed in the periodic table is an average of the atomic masses of all the naturally occurring isotopes of that element. This average is the atomic weight for that element.
Cite This Article
MLA
Deziel, Chris. "What Is Atomic Number?" sciencing.com, https://www.sciencing.com/what-is-atomic-number-13712134/. 26 April 2018.
APA
Deziel, Chris. (2018, April 26). What Is Atomic Number?. sciencing.com. Retrieved from https://www.sciencing.com/what-is-atomic-number-13712134/
Chicago
Deziel, Chris. What Is Atomic Number? last modified March 24, 2022. https://www.sciencing.com/what-is-atomic-number-13712134/