How To Write An Isotope
Some elements have only one naturally occurring isotope, but others have two, three or more. If you need to distinguish between the different isotopes of an element, you can represent each with a simple kind of notation that uses the mass number, the atomic symbol and the atomic number of the element. This notation is very easy to learn, although a little practice never hurts. Here's how to write isotopes for different elements.
Step 1
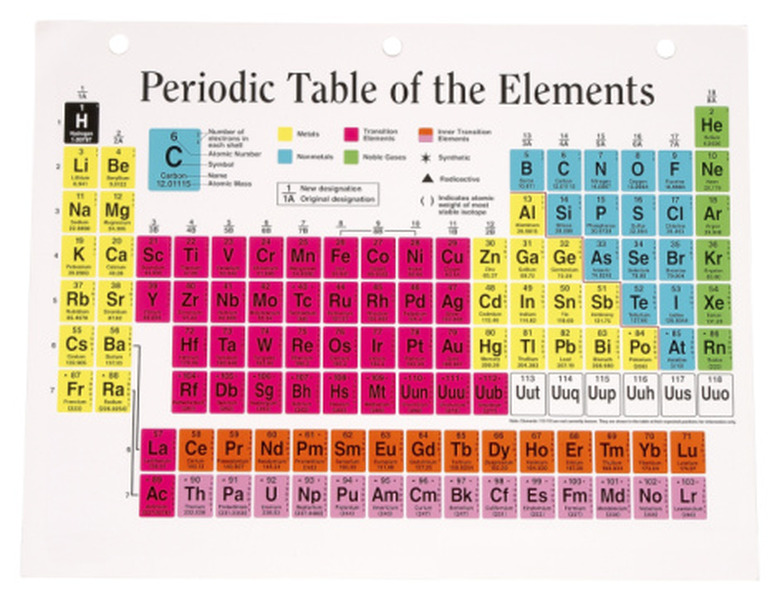
Look up the element you want to study on the periodic table and copy down its symbol. The symbol for carbon, for example, is a capital C.
Step 2
Find the atomic number or proton number for your element. This gives you the number of protons in its nucleus. This important number appears directly above the symbol for the element, and it will always be an integer. Carbon, for example, has an atomic number of 6.
Step 3
Write the atomic number as a subscript immediately preceding the symbol for your element. Click on the link under the Resources section if you want to see what this looks like.
Step 4
Write the mass number for your isotope as a superscript immediately preceding the symbol for the element. The mass number, in other words, goes directly above the number of protons.
Things Needed
- Pencil
- Paper
- Periodic table
- Mass number of your isotope
TL;DR (Too Long; Didn't Read)
Remember that the mass number is just the number of protons plus the number of neutrons.
Cite This Article
MLA
Brennan, John. "How To Write An Isotope" sciencing.com, https://www.sciencing.com/write-isotope-8381300/. 24 April 2017.
APA
Brennan, John. (2017, April 24). How To Write An Isotope. sciencing.com. Retrieved from https://www.sciencing.com/write-isotope-8381300/
Chicago
Brennan, John. How To Write An Isotope last modified March 24, 2022. https://www.sciencing.com/write-isotope-8381300/