You've Gotta Try This Fun, Easy Way To Grow Crystals At Home
We may receive a commission on purchases made from links.
Sciencing may earn compensation through affiliate links in this article.
Let's be honest: science experiments aren't exactly known for being pretty. Understandably, scientists care more about what they did during an experiment and what they learned – which means your home science lab probably isn't exactly TikTok-worthy.
But if you're craving a science project that's both educationally and aesthetically pleasing, crystal making is your jam. At-home crystal making kits make the process super easy, and could even be used to make fun, DIY decorations.
Ready to give it a try? Read on to learn all about crystals, how to grow them at home using a crystal growing kit, and other science experiments you might want to try after.
First, What Is a Crystal?
First, What Is a Crystal?
To put it simply, crystals are solid substances that have a standard and set geometric form. In plainer language, it's a substance whose atoms organize in a uniform geometric shape.
Look at the chemical structure of a crystal and you'll see something like a grid, where atoms are organized in a repeating pattern. As that pattern expands, it eventually forms a crystal shape.
There are four major types:
- **Ionic crystals:** As the name suggests, these are from positively- and negatively-charged ions. The crystal structure is held in place by ionic bonds. Some examples include table salt
- **Covalent network crystals:** These are made of atoms held together by covalent bonds. One example is diamonds, made from covalently-bonded carbon atoms attracted in a specific structure.
- **Metallic crystals:** Metallic crystals are made of metal atoms, like pure sodium, arranged into a crystal structure.
- **Molecular crystals:** These have a crystal structure formed from molecules. A classic example is ice: H20 frozen into a crystalline structure.
How Are Crystals Formed?
How Are Crystals Formed?
Crystals are found all over nature – and even in our cells and tissues. But since crystal growing science kits deal with ionic crystals, we'll focus on how those are formed.
Crystals are created when a solution that contains ions starts to cool down. Think back to to when you learned about saturated solutions. You know solutions can only hold a certain amount of solute, and the warmer the solution, the more solute you can dissolve in it.
So, when a solution cools down, it's not able to hold as many ions. As it gets colder, it becomes supersaturated – and eventually, ions start to precipitate out. These ions can latch onto any rough surface – even a piece of dust – and start forming a crystal. And as the solution keeps cooling, and more and more ions precipitate out, the crystals get bigger and bigger, until eventually you can see them with the naked eye.
What Gives Crystals Their Shape?
What Gives Crystals Their Shape?
A crystal's shape depends on the type of ions it contains, and how those ions interact with each other.
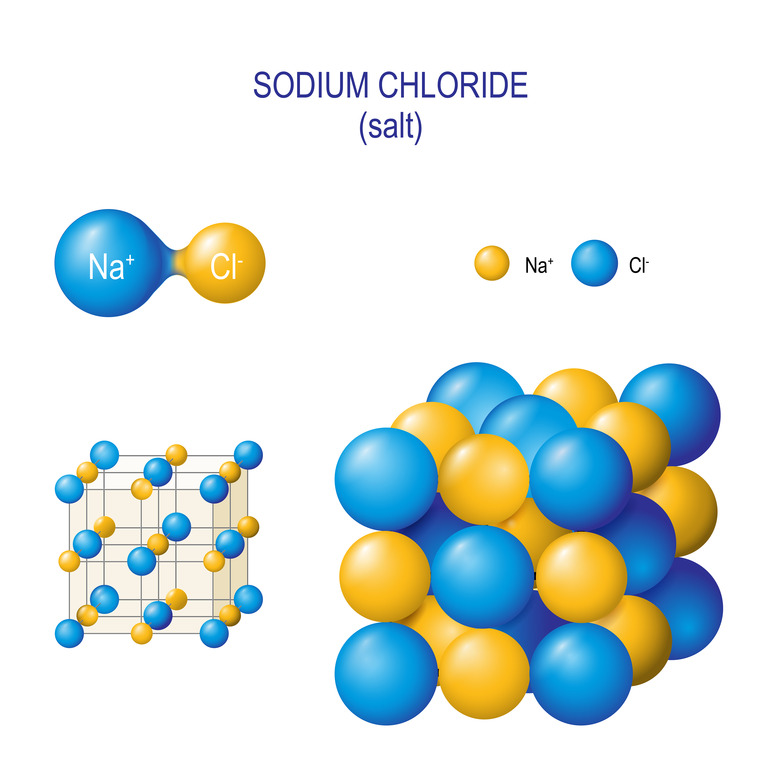
It's easiest to explain through an example, so let's look at table salt for a sec. Table salt is made of two types of ions – sodium and chloride – that sort themselves into a cube shape. Here's what it looks like on a microscopic level:
As you can see, the sodium and chloride atoms sort themselves into alternating patterns: each sodium ion is surrounded by chloride ion and vice versa. So if you looked at pure table salt under a microscope, you'd see a bunch of cube-shaped crystals.
But not ever crystal forms cubes. Depending on the atoms that make up the crystal, it can form several shapes.
Check out this blue crystal. It's clearly not a cube. That's because its chemical atoms form a hexagonal crystal – a hexagon shape with a pointed tip. This specimen has several crystal structures that formed around each other, which is how you get that "pointy" look.
Of course, different types of crystals can form even more complex structures. Think of DNA, a complex example of a molecular crystalline structure. It forms a double helix shape – a far cry from the cube formed by table salt.
Home crystal growing science experiments typically use table salt, so you can expect to see cube-like structures.
How to Do Your Own Crystal Growing Experiment
How to Do Your Own Crystal Growing Experiment
Making your own crystals at home is easy if you use a kit. We love [this crystal growing kit](https://www.etsy.com/listing/882445320/2x-crystal-growing-kits-fun-easy-and's safe and easy to do, and lets you observe two different types of crystal formations.
That's because the kit lets you grow salt crystals on two different surfaces. One allows you to grow crystals on carbon chunks. As the crystals form, they'll grow across the surface of the carbon, creating a crystal-covered rock.
The other allows you to grow crystals on a small cotton. The rough surfaces of the rope make an ideal place for crystals to form. As the crystal structure grows, it begins to branch out. So you'll end up with a tree-like shape with a rope "trunk" and crystal "branches."
In both cases, crystal formation takes time. But you'll start to see progress in a few hours, and your beautiful crystals will be finished growing in two to three days. Once they're formed, the crystals are fragile. But setting them in place with clear nail polish or hairspray may help them keep their shape.
Love Growing Crystals? Try These Other Science Activities!
Love Growing Crystals? Try These Other Science Activities!
If you're into crystals and geology (which is the study of rocks, btw), you'll love these other crystal and gem science experiments.
References
Conduct Your Own Crystal Dig
Archaeologists are experts at excavating historic sites – and you do your own version of a dig at home. This US&ref=as_li_ss_tl'>gemstone crystal dig kit contains 20 different types of minerals, like quartz and tiger's eye, plus tools to unearth them from the dig site.
Break Open Some Geodes
Geodes look like ordinary rocks – until you open them and see the crystals growing inside. US&ref=as_li_ss_tl'>This geode kit from National Geographic comes with 10 geodes, googles to help you break them open safely and a mini magnifying glass for closer inspection.
Make Glow Crystals
What's better than regular crystals? Crystals that glow, obviously. This US&ref=as_li_ss_tl'>glow crystal growing kit applies the same principles explained above, and allows to make glow-in-the-dark crystals within seven to 10 days.
Cite This Article
MLA
Tremblay, Sylvie. "You've Gotta Try This Fun, Easy Way To Grow Crystals At Home" sciencing.com, https://www.sciencing.com/crystal-growing-science-kit-13763612/. 31 March 2021.
APA
Tremblay, Sylvie. (2021, March 31). You've Gotta Try This Fun, Easy Way To Grow Crystals At Home. sciencing.com. Retrieved from https://www.sciencing.com/crystal-growing-science-kit-13763612/
Chicago
Tremblay, Sylvie. You've Gotta Try This Fun, Easy Way To Grow Crystals At Home last modified August 30, 2022. https://www.sciencing.com/crystal-growing-science-kit-13763612/