Golgi Apparatus: Function, Structure (With Analogy & Diagram)
Most people have built a cell model for a science fair or classroom science project, and few eukaryotic cell components are as interesting to look at or build as the Golgi apparatus.
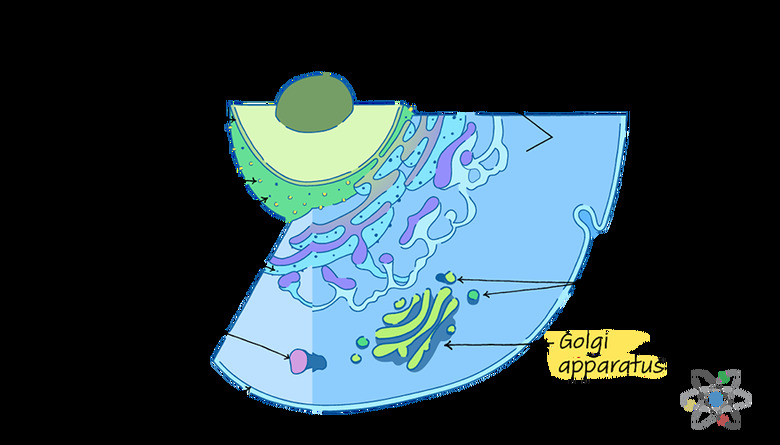
Unlike many organelles, which tend to have more uniform and often round shapes, the Golgi apparatus – also called the Golgi complex, Golgi body or even just Golgi – is a series of flat discs or pouches stacked together.
To the casual observer, the Golgi apparatus looks like a bird's eye view of a maze or maybe even a piece of ribbon candy.
This interesting structure helps the Golgi apparatus with its role as part of the endomembrane system, which comprises the Golgi body and a few other organelles, including the lysosomes and endoplasmic reticulum.
These organelles join together to alter, pack and transport important cell contents, such as lipids and proteins.
**Golgi apparatus analogy:** the Golgi apparatus is sometimes referred to as the packing plant or the post office of the cell because it receives molecules and makes changes to them then sorts and addresses those molecules for transport to other areas of the cell, just like a post office does with letters and packages.
Structure of the Golgi Body
Structure of the Golgi Body
The structure of the Golgi apparatus is crucial to its function.
Each of the flat pouches of membrane that stack together to form the organelle are called cisternae. In most organisms, there are four to eight of these discs, but some organisms can have up to 60 cisternae in a single Golgi body. The spaces in between each pouch are just as important as the pouches themselves.
These spaces are the Golgi apparatus' lumen.
Scientists divide the Golgi body into three parts: the cisternae close to the endoplasmic reticulum, which is the cis compartment; the cisternae far away from the endoplasmic reticulum, which is the trans compartment; and the middle cisternae, called the medial compartment.
These labels are important for understanding how the Golgi apparatus works because the outermost sides, or networks, of the Golgi body perform very different functions.
If you think of the Golgi apparatus as the cell's packing plant, you can visualize the cis side, or cis face, as the Golgi's receiving dock. Here, the Golgi apparatus takes in cargo sent from the endoplasmic reticulum through special transporters called vesicles.
The opposite side, called the trans face, is the shipping dock of the Golgi body.
Golgi Structure and Transport
Golgi Structure and Transport
After sorting and packaging, the Golgi apparatus releases proteins and lipids from the trans face.
The organelle loads the protein or lipid cargo into vesicle transporters, which bud off from the Golgi, destined for other places in the cell. For example, some cargo may go to the lysosome for recycling and degradation.
Other cargo might even wind up outside the cell after shipping to the cell's plasma membrane.
The cell's **cytoskeleton**, which is a matrix of structural proteins that give the cell its shape and help organize its contents, anchors the Golgi body in place near the endoplasmic reticulum and cell nucleus.
Since these organelles work together to build important biomolecules, such as proteins and lipids, it makes sense for them to set up shop in close proximity to one another.
Some of the proteins in the cytoskeleton, called **microtubules**, act like railroad tracks between these organelles as well as other locations within the cell. This makes it easy for transport vesicles to move cargo between the organelles and to their final destinations in the cell.
Enzymes: The Link Between Structure and Function
Enzymes: The Link Between Structure and Function
What happens in the Golgi between receiving the cargo at the cis face and shipping it out again at the trans face is some of the major work of the Golgi apparatus. The driving force behind this function is also driven by proteins.
The cisternae pouches in the various compartments of the Golgi body contain a special class of proteins called **enzymes**. The specific enzymes in each pouch enable it to modify the lipids and proteins as they pass from the cis face through the medial compartment on the way to trans face.
These modifications performed by the various enzymes in the cisternae pouches make a huge difference in the modified biomolecules' outcomes. Sometimes the modifications help make the molecules functional and able to do their jobs.
At other times, the modifications act like labels that inform the Golgi apparatus shipping center of the biomolecules' final destination.
These modifications affect the structure of the proteins and lipids. For example, enzymes might remove sugar side chains or add sugar, fatty acid or phosphate groups to the cargo.
Enzymes and Transport
Enzymes and Transport
The specific enzymes present in each of the cisternae determine which modifications happen in those cisternal pouches. For example, one modification cleaves the sugar mannose. This usually occurs in the earlier cis or medial compartments, based on the enzymes present there.
Another modification adds the sugar galactose or a sulfate group to the biomolecules. This generally happens near the end of the cargo's journey through the Golgi body in the trans compartment.
Since many of the modifications act like labels, the Golgi apparatus uses this information at the trans face to ensure that the newly altered lipids and proteins wind up at the correct destination. You can imagine this like a post office stamping packages with address labels and other shipping instructions for the mail handlers.
The Golgi body sorts the cargo based on those labels and loads the lipids and proteins into the appropriate vesicle transporters, ready to ship out.
Role in Gene Expression
Role in Gene Expression
Many of the alterations that take place in the cisternae of the Golgi apparatus are post-translational modifications.
These are changes made to proteins after the protein has already been built and folded. To make sense of this, you will need to travel backward in the scheme of protein synthesis.
Inside the nucleus of each cell, there is DNA, which acts like a blueprint for building biomolecules like proteins. The full set of DNA, called the human genome, contains both non-coding DNA and protein-coding genes. The information contained in each coding gene gives the instructions for building chains of amino acids.
Eventually, these chains fold into functional proteins.
However, this does not happen on a one-to-one scale. Since there are way, way more human proteins than there are coding genes in the genome, each gene must have the ability to produce multiple proteins.
Think of it this way: if scientists estimate that there are about 25,000 human genes and over 1 million human proteins, that means humans require over 40 times more proteins than they have individual genes.
Post-Translational Modifications
Post-Translational Modifications
The solution for building so many proteins from such a relatively small set of genes is post-translational modification.
This is the process by which the cell makes chemical modifications to the newly formed proteins (and older proteins at other times) in order to change what the protein does, where it localizes and how it interacts with other molecules.
There are a few common types of post-translational modification. These include phosphorylation, glycosylation, methylation, acetylation and lipidation.
- **Phosphorylation**: adds a phosphate group to the protein. This modification usually affects cell processes
related to cell growth and cell signaling.
- Glycosylation: occurs when the cell adds a sugar group to the protein. This modification is especially important for proteins
destined for the cell's plasma membrane or for secreted proteins, which wind up outside the cell.
- Methylation: adds a methyl group to the protein. This modification is a well-known _epigenetic regulator_. This basically means methylation can turn the influence of a gene
on or off. For example, people who experience a large-scale trauma, such as famine, pass on genetic changes to their children to help them survive future food shortages. One of the most common ways to pass those changes from one generation to another is through protein methylation.
- Acetylation: adds an acetyl group to the protein. The role of this modification isn't totally clear to researchers. However, they do know it is a common modification for **histones**, which are the proteins that act
as spools for the DNA.
- Lipidation: adds lipids to the protein. This makes the protein more opposed to water, or hydrophobic, and is very useful for proteins that are part of membranes.
Post-translational modification enables the cell to build a wide variety of proteins using a relatively small number of genes. These modifications change the way the proteins behave and therefore affect overall cell function. For instance, they may increase or decrease cell processes such as cell growth, cell death and cell signaling.
Some post-translational modifications affect cell functions related to human disease, so figuring out how and why modifications occur may help scientists develop medications or other treatments for these health conditions.
Role in Vesicle Formation
Role in Vesicle Formation
Once the modified proteins and lipids reach the trans face, they are ready for sorting and loading into the transport vesicles that will transport them to their final destinations in the cell. To do this, the Golgi body relies on those modifications that act as labels, telling the organelle where to send the cargo.
The Golgi apparatus loads the sorted cargo into vesicle transporters, which will bud off the Golgi body and travel to the final destination to deliver the cargo.
A vesicle sounds complex, but it is simply a bead of fluid surrounded by a membrane that protects the cargo during vesicular transport. For the Golgi apparatus, there are three types of transport vesicles: exocytotic vesicles, secretory vesicles and lysosomal vesicles.
Types of Vesicle Transporters
Types of Vesicle Transporters
Both exocytotic and secretory vesicles engulf the cargo and move it to the cell membrane for release outside the cell.
There, the vesicle fuses with the membrane and releases the cargo outside the cell through a pore in the membrane. Sometimes this happens immediately upon docking at the cell membrane. At other times, the transport vesicle docks at the cell membrane and then hangs out, waiting for signals from outside the cell before releasing the cargo.
A good example of exocytotic vesicle cargo is an antibody activated by the immune system, which needs to leave the cell in order to do its job to fight off pathogens. Neurotransmitters like adrenaline are a type of molecule that rely on secretory vesicles.
These molecules act like signals to help coordinate a response to a threat, such as during "fight or flight."
Lysosomal transport vesicles move cargo to the lysosome, which is the cell's recycling center. This cargo is generally damaged or old, so the lysosome strips it for parts and degrades the unwanted components.
The Golgi’s Function Is an Ongoing Mystery
The Golgi's Function Is an Ongoing Mystery
The Golgi body is no doubt a complex and a ripe area for ongoing research. In fact, even though the Golgi was first seen in 1897, scientists are still working on a model that fully explains how the Golgi apparatus functions.
One area of debate is how exactly the cargo moves from the cis face to the trans face.
Some scientists think that vesicles carry the cargo from one cisterna pouch to the next. Other researchers think the cisternae themselves move, maturing as they move from the cis compartment to the trans compartment and carrying the cargo with them.
The latter is the maturation model.
Cite This Article
MLA
Mayer, Melissa. "Golgi Apparatus: Function, Structure (With Analogy & Diagram)" sciencing.com, https://www.sciencing.com/golgi-apparatus-function-structure-with-analogy-diagram-13717291/. 15 March 2019.
APA
Mayer, Melissa. (2019, March 15). Golgi Apparatus: Function, Structure (With Analogy & Diagram). sciencing.com. Retrieved from https://www.sciencing.com/golgi-apparatus-function-structure-with-analogy-diagram-13717291/
Chicago
Mayer, Melissa. Golgi Apparatus: Function, Structure (With Analogy & Diagram) last modified August 30, 2022. https://www.sciencing.com/golgi-apparatus-function-structure-with-analogy-diagram-13717291/